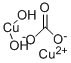
Cupric carbonate basic
- CAS No.
- 12069-69-1
- Chemical Name:
- Cupric carbonate basic
- Synonyms
- COPPER CARBONATE;basic;CUPRIC CARBONATE;Basic copper carbonate;basiccopper(ii)carbonate;COPPER(II) CARBONATE BASIC;copper(ii)carbonatehydroxide(2:1:2);CuCO;Artificialmalachite;COPPER (II) CARBONATE
- CBNumber:
- CB9852923
- Molecular Formula:
- CO3.Cu.CuH2O2
- Molecular Weight:
- 221.11
- MDL Number:
- MFCD00010976
- MOL File:
- 12069-69-1.mol
- MSDS File:
- SDS
| Melting point | 200 °C |
|---|---|
| Density | 4 |
| storage temp. | Sealed in dry,Room Temperature |
| solubility | Aqueous Acid (Slightly) |
| form | Solid |
| Specific Gravity | 4. |
| color | green |
| Water Solubility | Insoluble |
| Merck | 14,2631 |
| Solubility Product Constant (Ksp) | pKsp: 9.86 |
| Exposure limits |
ACGIH: TWA 1 mg/m3 NIOSH: IDLH 100 mg/m3; TWA 1 mg/m3 |
| FDA 21 CFR | 582.80 |
| CAS DataBase Reference | 12069-69-1(CAS DataBase Reference) |
| FDA UNII | GIK928GH0Y |
| Pesticides Freedom of Information Act (FOIA) | Copper carbonate |
| EPA Substance Registry System | Copper carbonate, basic (12069-69-1) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS07,GHS09 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H302+H332-H319-H410 | |||||||||
| Precautionary statements | P261-P264-P273-P301+P312-P304+P340+P312-P305+P351+P338 | |||||||||
| Hazard Codes | Xn,N | |||||||||
| Risk Statements | 22-36/37/38-50/53 | |||||||||
| Safety Statements | 26-36-61-60 | |||||||||
| RIDADR | UN3288 | |||||||||
| WGK Germany | 2 | |||||||||
| RTECS | GL6910000 | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 6.1 | |||||||||
| PackingGroup | III | |||||||||
| HS Code | 28369911 | |||||||||
| Toxicity | bird - domestic,LD50,oral,900mg/kg (900mg/kg),Pesticide Chemicals Official Compendium, Association of the American Pesticide Control Officials, Inc., 1966. Vol. -, Pg. 258, 1966. | |||||||||
| NFPA 704 |
|
Cupric carbonate basic price More Price(29)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 61167 | Copper(II) carbonate basic purum p.a., ≥95% (RT) | 12069-69-1 | 250g | $94 | 2023-06-20 | Buy |
| Sigma-Aldrich | 61167 | Copper(II) carbonate basic purum p.a., ≥95% (RT) | 12069-69-1 | 1kg | $240 | 2023-06-20 | Buy |
| Alfa Aesar | 033305 | Copper(II) carbonate dihydroxide, Cu 55% min | 12069-69-1 | 500g | $72.9 | 2024-03-01 | Buy |
| Alfa Aesar | 033305 | Copper(II) carbonate dihydroxide, Cu 55% min | 12069-69-1 | 2kg | $205 | 2024-03-01 | Buy |
| Strem Chemicals | 93-2911 | Copper(II) carbonate, basic | 12069-69-1 | 500g | $62 | 2024-03-01 | Buy |
Cupric carbonate basic Chemical Properties,Uses,Production
Malachite
Cupric carbonate basic exhibits the color of malachite green, thus being called malachite, being a precious mineral gem. It is product produced from the reaction between copper and the oxygen, carbon dioxide and water in the air, also known as copper rust with green color. When being heated in the air, it will be broken down into copper oxide, water and carbon dioxide. It is soluble in acid and can generate the corresponding copper salt. It is also soluble in the aqueous solution of cyanide, ammonium salt and alkali metal carbonate to form copper complex. When being boiled in water or heated in alkaline solution, it can generate brown copper oxide. At 200 ℃, it can be decomposed into black copper oxide. It is very unstable under hydrogen sulfide atmosphere and can have reaction with hydrogen sulfide to generate copper sulfide. Cupric carbonate basic, according to the ratio difference of CuCO3 over H2O, has a dozen different forms of compounds. It is existed in the form of malachite in nature in the form of malachite.
When being placed in the air for a long time, it will absorb moisture and release carbon dioxide, slowly turning into the green malachite composition. In nature, it is existed in the form of blue copper.
Copper carbonate and cupric carbonate basic are actually not presented. The addition of sodium carbonate to the dilute copper sulfate solution, or the introduction of carbon dioxide into the suspension of copper hydroxide can both give the precipitate of cupric carbonate basic. Cupric carbonate basic can be seen as consisting of the copper hydroxide and copper carbonate. Actually there are two types of copper hydroxides with both combination with one copper carbonate and two copper carbonates.
The former one has the chemical formula of CuCO3 • Cu (OH) 2, being a monoclinic crystal fiber-like clusters, or dark green powder. The resulting precipitate from the solution was initially green and, after standing, turned dark green in solution. It is toxic, being the major components in the green rust (commonly known as copper green) generated in the copper surface.
The latter one has a chemical formula of 2CuCO3 • Cu (OH) 2, being dark sky blue, being very bright monoclinic crystal, or compact crystal clumps. It is insoluble in water, soluble in ammonia and hot and concentrated sodium bicarbonate solution and can turn into blue color. It can be decomposed at 300 °C.
Basic copper carbonate can be used to manufacture signal flares, pyrotechnics, paint pigments, other copper salts, solid phosphor activators, pesticides, seed treatment and as fungicides and antidotes as well as used for plating and so on.
Solubility in water (g / 100ml)
Solubility in 100 ml of water (grams):
1.462 × 10-4/20 ° C
Toxicity
For harmful effects of excess amount of copper, the reaction between Cu2+ with the enzyme hydrogen sulfide plays a central role, drinking of water containing 44 mg /L copper can lead to the occurrence of acute gastroenteritis. Oral administration of copper salt for 0.2~0.5 g can cause vomiting with l~2 g being able to cause severe vomiting and sometimes can lead to fatal poisoning. Chronic poisoning is manifested as nervous system dysfunction, liver and kidney dysfunction, nasal septum ulcers and perforation. The facial skin, hair and conjunctiva sometimes can become light yellow green or light green. Dust and solution can both irritate the eyes and mucous membranes.
The maximum allowable concentration of copper metal is 1 mg/m3; for copper oxide, the value is 0.1 mg/m3.
Upon copper poisoning, the patients can be subject to oral treatment with 0.1% K4 [Fe (CN) 6] solution for gastric lavage, or oral administration of protein water, magnesia laxative; upon abdominal paining, the patients can be subject to subcutaneous injection of 1 mL of 0.1% atropine sulfate; upon eye irritation, we can use water to rinse.
Upon working, we should wear protective masks, protective glasses and dust uniforms.
Chemical properties
It appears as peacock green small amorphous powder. It can’t be dissolved in cold water and alcohol, being soluble in acid to form the corresponding copper salt. It is soluble in the cyanide, ammonia, ammonium salt and alkali metal carbonate aqueous solution.
Uses
It can be applied to the fields of pyrotechnics, pesticides, pigments, feed, fungicides, preservatives and other industries for the manufacturing of copper compounds.
It can be applied to analysis reagents and pesticides
It can be applied to organic catalysts, pyrotechnics and pigments. In the field of agriculture, it can be used for the prevention of plants smut, as the poisoning antidote for insecticides and phosphorus poison antidote as well as the germicide of the seeds; being mixed with asphalt can prevent animal husbandry and wild rat from eating seedlings; it can be applied to feed as the copper additive. In the crude oil storage, it can be used as alkali agent and the raw material for the production of copper compounds. It can also be used for electroplating, corrosion and analysis reagents.
It can be applied to paint color, pyrotechnics, pesticides, seed treatment germicide and for preparation of other copper salts. It can also be used as solid fluorescent powder activator.
Production method
Copper sulfate method; formulate the baking soda into solution of relative density of 1.05, first add to the reactor, at 50 ° C, under stirring, add refined copper sulfate solution and control the reaction temperature of 70~80 ° C. The reaction takes the change of the precipitate color to peacock green as the turning point with the pH being maintained at 8. After the stopping of the reaction, after standing and sedimentation, use 70~80 ℃ water or no ion-water to wash to until the lotion has no SO42-anymore, followed by centrifugal separation, drying to obtain the finished product of cupric carbonate base. The reaction is:
2CuSO4 + 4NaHCO3 → CuCO3 • Cu (OH) 2 + 2Na2SO4 + 3CO2 ↑ + H2O
Copper nitrate: after the electrolytic copper is reacted with concentrated nitric acid to produce copper nitrate, and then have reaction with the mixture solution of sodium carbonate and sodium bicarbonate to generate cupric carbonate basic. The precipitate is subjecting to washing, separation and dehydration, drying to yield the final products. The reaction processes are:
Cu + 4HNO3 → Cu (NO3) 2 + 2NO2 ↑ + 2H2O
2Cu (NO3) 2 + 2Na2CO3 + H2O → CuCO3 • Cu (OH) 2 + 4NaNO3 + CO2 ↑
2Cu (NO3) 2 + 4NaHCO3 → CuCO3 • Cu (OH) 2 + 4NaNO3 + 3CO2 ↑ + H2O
Category
Pesticides
Toxic grading
poisoning
Acute Toxicity
Oral-Rat LD50: 1350 mg/kg
Flammability and hazardous properties
it is non-combustible with combustion yielding toxic copper-containing fumes;
Storage and transport characteristics
Treasury at low temperature, ventilation, dry
Fire extinguishing agent
water, carbon dioxide, dry powder, and sand
Professional Standard
TWA 1 mg/m3; STEL 3 mg/m3
Chemical Properties
Copper Carbonate (Basic), CuCO3·Cu(OH)2, darkgreen monoclinic crystals, insoluble in cold H2O decomposes in hot H2O, soluble in potassium cyanide. Malachite, a copper ore, is of this composition. Refined compound is used as a pigment.
Chemical Properties
Copper carbonate (basic), dark green monoclinic crystals, insoluble in cold H20, decomposes in H20, soluble in potassium cyanide. Malachite, copper ore, is of this composition. Refmed compound is used as a pigment.
Physical properties
Natural malachite is a dark green crystalline solid; monoclinic crystals; density 4.0 g/cm3; refractive index 1.655; decomposes at 200°C; insoluble in cold water and alcohols; decomposes in hot water; soluble in acids, ammonium hydroxide and potassium cyanide solutions. Natural azurite is blue monoclinic crystal; density 3.88 g/cm3shiz; refractive index 1.730; decomposes at 220°C; insoluble in cold water; decomposes in hot water; soluble in ammonium hydroxide and hot sodium bicarbonate solutions.
Uses
As seed treatment fungicide; in pyrotechnics; as paint and varnish pigment; in animal and poultry feeds; in sweetening of petrol sour crude stock; in manufacture of other Cu salts.
Uses
Copper(II) carbonate dihydroxide is used as pigments and in treatment for copper deficiency in ruminants. It is also used in pyrotechnics, in sweetening of petrol sour crude stock and animal and poultry feeds. It finds application in some types of make-up such as lipstick. In analytical chemistry, it is used as a reagent for analysis.
Uses
Used to kill algae and as a pigment
Preparation
Basic carbonate is obtained from its naturally occurring minerals. It also may be prepared by mixing a solution of copper sulfate with sodium carbonate. The precipitate is then filtered and dried.
General Description
Copper(II) carbonate basic is naturally found in azurite and malachite. On heating, it undergoes decomposition to form copper(II) oxide, carbon dioxide and water. Due to its high initial discharge capacity, copper(II) carbonate (basic) is being considered as a promising candidate as anode materials for lithium-ion batteries.
Hazard
Toxic by ingestion.
Cupric carbonate basic Preparation Products And Raw materials
Raw materials
1of2
Preparation Products
1of2
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 | sales@hbmojin.com | China | 12453 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21695 | 55 |
| Shanghai Yingrui Biopharma Co., Ltd. | +86-21-33585366 - 03@ | sales03@shyrchem.com | CHINA | 738 | 60 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418679 +86-18949832763 | info@tnjchem.com | China | 2989 | 55 |
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
| SHANDONG ZHI SHANG CHEMICAL CO.LTD | +86 18953170293 | sales@sdzschem.com | China | 2931 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Hebei Guanlang Biotechnology Co., Ltd. | +86-19930503282 | alice@crovellbio.com | China | 8822 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 | linda@hubeijusheng.com | CHINA | 22968 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-61398051 +8613650506873 | sales@chemdad.com | China | 39916 | 58 |
View Lastest Price from Cupric carbonate basic manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2023-09-21 | Cupric carbonate basic
12069-69-1
|
US $0.00 / KG | 1KG | 99% | 50000KG/month | Hebei Mojin Biotechnology Co., Ltd | |
 |
2021-07-24 | Cuco3 Powder Copper (II) Carbonate Basic
12069-69-1
|
US $3700.00 / KG | 500KG | 99% | 5000 | Hebei Henghe Import and Export Trading Co. LTD | |
 |
2021-07-13 | Cupric carbonate basic
12069-69-1
|
US $15.00-10.00 / KG | 1KG | 99%+ HPLC | Monthly supply of 1 ton | Zhuozhou Wenxi import and Export Co., Ltd |
-

- Cupric carbonate basic
12069-69-1
- US $0.00 / KG
- 99%
- Hebei Mojin Biotechnology Co., Ltd
-

- Cuco3 Powder Copper (II) Carbonate Basic
12069-69-1
- US $3700.00 / KG
- 99%
- Hebei Henghe Import and Export Trading Co. LTD
-

- Cupric carbonate basic
12069-69-1
- US $15.00-10.00 / KG
- 99%+ HPLC
- Zhuozhou Wenxi import and Export Co., Ltd