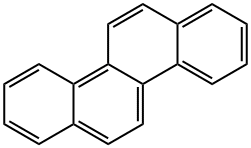
Chrysene
- CAS No.
- 218-01-9
- Chemical Name:
- Chrysene
- Synonyms
- CHRYSEN;[4]Phenacene;BENZO[A]PHENANTHRENE;1,2-Benzophenanthrene;⋲Crysene;Chrycene;NSC 6175;CHRYSENE;Chrysene,98%
- CBNumber:
- CB9853344
- Molecular Formula:
- C18H12
- Molecular Weight:
- 228.29
- MDL Number:
- MFCD00003698
- MOL File:
- 218-01-9.mol
- MSDS File:
- SDS
| Melting point | 252-254 °C (lit.) |
|---|---|
| Boiling point | 448 °C (lit.) |
| Density | 1.274 |
| vapor pressure | 4.3 at 25 °C (de Kruif, 1980) |
| refractive index | 1.7480 (estimate) |
| Flash point | -17℃ |
| storage temp. | Store below +30°C. |
| solubility | <0.0001g/l |
| color | White to Light yellow to Light orange |
| Water Solubility | insoluble |
| Merck | 14,2255 |
| BRN | 1909297 |
| Henry's Law Constant | 1.97, 6.91, 18.8, 52.3, and 118 at 4.1, 11.0, 18.0, 25.0, and 31.0 °C, respectively (Bamford et al., 1998) |
| Stability | Stable. Combustible. Incompatible with strong oxidizing agents. |
| InChIKey | WDECIBYCCFPHNR-UHFFFAOYSA-N |
| CAS DataBase Reference | 218-01-9(CAS DataBase Reference) |
| EWG's Food Scores | 4 |
| FDA UNII | 084HCM49PT |
| Proposition 65 List | Chrysene |
| NIST Chemistry Reference | Chrysene(218-01-9) |
| IARC | 2B (Vol. 92) 2010 |
| EPA Substance Registry System | Chrysene (218-01-9) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS08,GHS09 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H341-H350-H410 | |||||||||
| Precautionary statements | P201-P273-P308+P313 | |||||||||
| Hazard Codes | T,N,Xn,F | |||||||||
| Risk Statements | 45-50/53-68-40-67-66-36-11-52/53-36/37/38 | |||||||||
| Safety Statements | 53-45-60-61-36/37-26-16-24/25-23 | |||||||||
| RIDADR | UN 3077 9/PG 3 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | GC0700000 | |||||||||
| HazardClass | 9 | |||||||||
| PackingGroup | III | |||||||||
| HS Code | 29029090 | |||||||||
| Toxicity | Acute LC50 for Neanthes arenaceodentata >50 μg/L (Rossi and Neff, 1978). | |||||||||
| NFPA 704 |
|
Chrysene price More Price(17)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 8.41690 | Chrysene for synthesis | 218-01-9 | 1G | $189 | 2024-03-01 | Buy |
| Sigma-Aldrich | 40074 | Chrysene solution certified reference material, 1000?μg/mL in acetone | 218-01-9 | 1mL | $16.76 | 2024-03-01 | Buy |
| Sigma-Aldrich | 35754 | Chrysene analytical standard | 218-01-9 | 100mg | $130 | 2024-03-01 | Buy |
| TCI Chemical | C0339 | Benzo[a]phenanthrene (purified by sublimation) >98.0%(GC) | 218-01-9 | 1g | $282 | 2024-03-01 | Buy |
| TCI Chemical | C0339 | Benzo[a]phenanthrene (purified by sublimation) >98.0%(GC) | 218-01-9 | 100mg | $94 | 2024-03-01 | Buy |
Chrysene Chemical Properties,Uses,Production
Description
Chrysene is a polycyclic aromatic hydrocarbon (PAH) consisting of four fused benzene rings. Some of its derivatives such as electroluminescent 3, 6, 9, 12-tetrasubstituted chrysenes are useful in electroluminescent applications such as being used in the organic light emitting dioxide (OLED). They are also relates to electronic devices in which the active layer includes such a chrysene composition. Chrysene is a potential carcinogen.
Sources
Tokito, Shizuo, et al. Applied Physics Letters 77.2(2000):160-162.
Gao, Weiying, D. T. Deibler, and V. Rostovtsev. "Chrysene derivative host materials." US, US8932733. 2015.
Ionkin, Alex Sergey. "Tetra-substituted chrysenes for luminescent applications." US, US8115378. 2012.
https://en.wikipedia.org/wiki/Chrysene
Description
Chrysene is a polycyclic aromatic hydrocarbon (PAH) with the molecular formula C18H12. It is one of the natural constituents in coal tar, from which it was first isolated and characterized. It is produced as a gas during combustion of coal, gasoline, garbage, animal, and plant materials and usually found in smoke and soot. Chrysene usually combines with dust particles in the air and is carried into water and soil and onto crops. Creosote, a chemical used to preserve wood contains chrysene. High concentration of chrysene in the air is typically found during open burning and home heating with wood and coal. People are exposed to chrysene from a variety of environmental sources such as air, water, and soil and from cigarette smoke and cooked food. General population is usually exposed to chrysene along with a mixture of similar chemicals. Chrysene is a by-product of many industrial processes and thereby released in the atmosphere. Chrysene is lipophilic, insoluble in water, slightly soluble in other polar solvents such as alcohol, ether and moderately soluble in benzene and toluene. However, it readily dissolves in benzene and toluene at an elevated temperature. The name ‘Chrysene’ originates from the Greek word chrysos, meaning ‘gold,’ and is due to the golden yellow color of the slightly impure crystals. However, in pure state, chrysene is a colorless, crystalline solid. It has characteristic red–blue fluorescence under UV light. Some important properties of chrysene are summarized below.
Chemical Properties
crystalline powder
Chemical Properties
Chrysene is a combustible, white (when pure), red, or blue, fluorescent crystalline solid. Odorless. Chrysene 859 Polycyclic aromatic hydrocarbons (PAHs) are compounds containing multiple benzene rings and are also called polynuclear aromatic hydrocarbons
Physical properties
Orthorhombic, bipyramidal plates from benzene exhibiting strong reddish-blue fluorescence under UV light
Uses
Used strictly for research purposes.
Uses
Laboratory reagent; formed during the pyrolysis of organic matter
Uses
Organic synthesis.
Uses
Chrysene may be used as an analytical reference standard for the determination of the analyte in fish bile, air particulate extracts and food samples by various chromatography techniques.
Definition
ChEBI: Chrysene is an ortho-fused polycyclic arene found commonly in the coal tar. It has a role as a plant metabolite.
Synthesis Reference(s)
Tetrahedron Letters, 29, p. 3865, 1988 DOI: 10.1016/S0040-4039(00)82136-X
General Description
A crystalline solid. Denser than water and insoluble in water. The primary hazard is the threat to the environment. Immediate steps should be taken to limit spread to the environment. Toxic by ingestion. Used to make other chemicals.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
Vigorous reactions, sometimes amounting to explosions, can result from the contact between aromatic hydrocarbons, such as Chrysene, and strong oxidizing agents. They can react exothermically with bases and with diazo compounds. Substitution at the benzene nucleus occurs by halogenation (acid catalyst), nitration, sulfonation, and the Friedel-Crafts reaction.
Hazard
Possible carcinogen.
Health Hazard
There is very little information published onthe acute toxicity of chrysene. The oral toxicity is expected to be low. Animal studies showsufficient evidence of carcinogenicity. It produced skin cancer in animals. Subcutaneousadministration of chrysene in mice causedtumors at the site of application. Cancer-causing evidence in humans is not known. Ahistidine reversion–Ames test for mutagenicity showed positive.
Health Hazard
ACUTE/CHRONIC HAZARDS: Toxic.
Fire Hazard
Some may burn but none ignite readily. Containers may explode when heated. Some may be transported hot.
Safety Profile
Confirmed carcinogen with experimental carcinogenic, neoplastigenic, and tumorigenic data by skin contact. Human mutation data reported. When heated to decomposition it emits acrid smoke and fumes.
Potential Exposure
Almost never found by itself, chrysene is found in gasoline and diesel exhaust as well as in cigarette smoke; and in coal tar; coal tar pitch; creosote. It is used in organic synthesis.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit.
Carcinogenicity
The IARC has determined that there is limited evidence that chrysene is carcinogenic to experimental animals.ACGIH has classified chrysene as a confirmed animal carcinogen with unknown relevance to humans; a numerical threshold limit value (TLV) is not recommended.
Source
Identified in Kuwait and South Louisiana crude oils at concentrations of 6.9 and 17.5
ppm, respectively (Pancirov and Brown, 1975). Also present in high octane gasoline (6.7 mg/kg),
bitumen (1.64–5.14 ppm), gasoline exhaust (27–318 μg/m3), cigarette smoke (60 μg/1,000
cigarettes), and South Louisiana crude oil (17.5 ppm) (quoted, Verschueren, 1983). Also detected
in fresh motor oil (56 mg/L), used motor oil (10.17 mg/L) (Pasquini and Monarca, 1093).
Detected in groundwater beneath a former coal gasification plant in Seattle, WA at a
concentration of 10 μg/L (ASTR, 1995). The concentration of chrysene in coal tar and the
maximum concentration reported in groundwater at a mid-Atlantic coal tar site were 3,600 and 0.0063 mg/L, respectively (Mackay and Gschwend, 2001). Based on laboratory analysis of 7 coal
tar samples, chrysene concentrations ranged from 620 to 5,100 ppm (EPRI, 1990). Chrysene was
also detected in 9 commercially available creosote samples at concentrations ranging from 19 to
620 mg/kg (Kohler et al., 2000).
Identified in high-temperature coal tar pitches used in roofing operations at concentrations
ranging from 2,600 to 88,000 mg/kg (Arrendale and Rogers, 1981; Malaiyandi et al., 1982).
Chrysene was detected in asphalt fumes at an average concentration of 115.67 ng/m3 (Wang et
al., 2001).
Under atmospheric conditions, a low rank coal (0.5–1 mm particle size) from Spain was burned
in a fluidized bed reactor at seven different temperatures (50 °C increments) beginning at 650 °C.
The combustion experiment was also conducted at different amounts of excess oxygen (5 to 40%)
and different flow rates (700 to 1,100 L/h). At 20% excess oxygen and a flow rate of 860 L/h, the
amount of chrysene emitted ranged from 127.9 ng/kg at 950 °C to 1,186.0 ng/kg at 750 °C. The
greatest amount of PAHs emitted were observed at 750 °C (Mastral et al., 1999).
Environmental Fate
Biological. When chrysene was statically incubated in the dark at 25 °C with yeast extract and
settled domestic wastewater inoculum, significant biodegradation with varied adaptation rates was
observed. At concentrations of 5 and 10 mg/L, 59 and 38% biodegradation, respectively, were
observed after 28 d (Tabak et al., 1981).
Soil. The reported half-lives for chrysene in a Kidman sandy loam and McLaurin sandy loam
are 371 and 387 d, respectively (Park et al., 1990).
Surface Water. In a 5-m deep surface water body, the calculated half-lives for direct
photochemical transformation at 40 °N latitude, in the midsummer during midday were 13 h and
68 d with and without sediment-water partitioning, respectively (Zepp and Schlotzhauer, 1979).
Photolytic. Based on structurally related compounds, chrysene may undergo photolysis to yield
quinones (U.S. EPA, 1985) and/or hydroxy derivatives (Nielsen et al., 1983). The atmospheric
half-life was estimated to range from 0.802 to 8.02 h (Atkinson, 1987). Behymer and Hites (1985)
determined the effect of different substrates on the rate of photooxidation of chrysene using a
rotary photoreactor. The photolytic half-lives of chrysene using silica gel, alumina, and fly ash
were 100, 78, and 38 h, respectively.
storage
Color Code—Green: General storage may be used.Prior to working with chrysene you should be trained on itsproper handling and storage. A regulated, marked areashould be established where this chemical is handled, used,or stored in compliance with OSHA Standard 1910.1045.Store in tightly closed containers in a cool, well-ventilatedarea away from oxidizers. Sources of ignition, such assmoking and open flames, are prohibited where this chemical is used, handled, or stored in a manner that could createa potential fire or explosion hazard.
Shipping
UN3077 Environmentally Hazardous substances, solid, n.o.s., Hazard class: 9; Labels: 9-Miscellaneous hazardous material, Technical Name Required.
Purification Methods
Purify chrysene by chromatography on alumina from pet ether in a darkened room. Its solution in *C6H6 is passed through a column of decolorising charcoal, then crystallised by concentrating the eluate. It has also been purified by crystallising from *C6H6 or *C6H6/pet ether, and by zone refining. [Gorman et al. J Am Chem Soc 107 4404 1985]. It is freed from 5H-benzo[b]carbazole by dissolving it in N,N-dimethylformamide and successively adding small portions of alkali and iodomethane until the fluorescent colour of the carbazole anion no longer appears when alkali is added. The chrysene (and alkylated 5H-benzo[b]carbazole) separate on addition of water. Final purification is by crystallisation from ethylcyclohexane and/or from 2-methoxyethanol [Bender et al. Anal Chem 36 1011 1964]. It can be sublimed in a vacuum. [Beilstein 5 IV 2554.]
Toxicity evaluation
Generally, disposal of PAH from the industrial plants, accidental release from the containers, smoke from plant, combustion, or automobile exhaust causes chrysene and other PAHs to enter the environment. Because of the poor water solubility and low vapor pressure, chrysene has limited chance to get washed away or evaporate in the environment. Therefore, it remains immobile in soils. If exposed to water, it gets absorbed on the particulate matters and either float or sediment on the riverbed. The rate of biodegradation in soil ranges from 77 to 387 days depending on the soil type.Chrysene does not undergo hydrolysis due to the lack of hydrolyzable functional groups. However, it undergoes photochemical oxidations when exposed to the environment. Dihydrodiol is the common degradation product of chrysene. Half-life of degradation of chrysene, absorbed to soot particles and exposed to sunlight in air containing 10 ppm nitrogen oxides is 26 days. The National Research Council (NRC 1983) noted that the PAHs adsorbed to soot particles are more resistant to photochemical reactions than pure compounds.
Incompatibilities
Contact with strong oxidizers may cause fire and explosion hazard
Waste Disposal
Chrysene may be destroyed by permanganate oxidation, by high-temperature incinerator with scrubbing equipment; or by microwave plasma treatment.
Chrysene Preparation Products And Raw materials
Raw materials
1of3
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Springchem New Material Technology Co.,Limited | +86-021-62885108 +8613917661608 | info@spring-chem.com | China | 2068 | 57 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21695 | 55 |
| ATK CHEMICAL COMPANY LIMITED | +undefined-21-51877795 | ivan@atkchemical.com | China | 32480 | 60 |
| Shanghai Zheyan Biotech Co., Ltd. | 18017610038 | zheyansh@163.com | CHINA | 3620 | 58 |
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
| City Chemical LLC | 2039322489 | sales@citychemical.com | United States | 168 | 58 |
| TianYuan Pharmaceutical CO.,LTD | +86-755-23284190 13684996853 | sales@tianpharm.com | CHINA | 304 | 58 |
| Jinan Finer Chemical Co., Ltd | +86-531-88989536 +86-15508631887 | sales@finerchem.com | China | 2967 | 58 |
| Accela ChemBio Inc. | (+1)-858-699-3322 | info@accelachem.com | United States | 19965 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-61398051 +8613650506873 | sales@chemdad.com | China | 39916 | 58 |
Related articles
- Toxicity of Chrysene
- Chrysene is a polycyclic aromatic hydrocarbon (PAH) with the molecular formula C18H12. It is one of the natural constituents i....
- Jan 11,2022
View Lastest Price from Chrysene manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2021-07-13 | Chrysene
218-01-9
|
US $15.00-10.00 / KG | 1KG | 99%+ HPLC | Monthly supply of 1 ton | Zhuozhou Wenxi import and Export Co., Ltd | |
 |
2021-07-09 | Chrysene
218-01-9
|
US $15.00-10.00 / KG | 1KG | 99%+ HPLC | Monthly supply of 1 ton | Zhuozhou Wenxi import and Export Co., Ltd | |
 |
2019-07-06 | Chrysene
218-01-9
|
US $2.00 / kg | 1kg | 99% | ask | Career Henan Chemical Co |