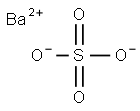Barium sulfate
- CAS No.
- 7727-43-7
- Chemical Name:
- Barium sulfate
- Synonyms
- Precipitated BariuM Sulfate;BLANC FIXE;BARYTE;e-z-hd;Barium sulphate, precipitated;hd85;epi-c;ba147;rugar;enecat
- CBNumber:
- CB9854295
- Molecular Formula:
- BaO4S
- Molecular Weight:
- 233.39
- MDL Number:
- MFCD00003455
- MOL File:
- 7727-43-7.mol
- MSDS File:
- SDS
| Melting point | 1580 °C |
|---|---|
| Boiling point | decomposes at 1580℃ [KIR78] |
| Density | 4.5 |
| storage temp. | Storage temperature: no restrictions. |
| solubility | water: insoluble |
| form | powder |
| Specific Gravity | 4.5 |
| color | White to yellow |
| PH | 3.5-10.0 (100g/l, H2O, 20℃)suspension |
| PH Range | 7 |
| Odor | wh. or ylsh. fine powd. free from grittiness, odorless, tasteless |
| Water Solubility | 0.0022 g/L (50 ºC) |
| Merck | 14,994 |
| Solubility Product Constant (Ksp) | pKsp: 9.97 |
| Exposure limits |
ACGIH: TWA 5 mg/m3 OSHA: TWA 15 mg/m3; TWA 5 mg/m3 NIOSH: TWA 10 mg/m3; TWA 5 mg/m3 |
| Dielectric constant | 11.4(16.0℃) |
| Stability | Stable. |
| LogP | -1.031 (est) |
| CAS DataBase Reference | 7727-43-7(CAS DataBase Reference) |
| Indirect Additives used in Food Contact Substances | BARIUM SULFATE |
| FDA 21 CFR | 175.105; 176.170; 177.2600; 178.3297 |
| EWG's Food Scores | 2 |
| NCI Dictionary of Cancer Terms | barium sulfate; barium swallow |
| FDA UNII | 25BB7EKE2E |
| EPA Substance Registry System | Barium sulfate (7727-43-7) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H315-H319 | |||||||||
| Precautionary statements | P264-P280-P302+P352-P337+P313-P305+P351+P338-P362+P364-P332+P313 | |||||||||
| Hazard Codes | Xn | |||||||||
| Risk Statements | 20/21/22-36/37/38 | |||||||||
| Safety Statements | 22-24/25-36-26 | |||||||||
| WGK Germany | - | |||||||||
| RTECS | CR0600000 | |||||||||
| TSCA | Yes | |||||||||
| HS Code | 28332700 | |||||||||
| Toxicity | LD50 orally in Rabbit: > 20000 mg/kg | |||||||||
| NFPA 704 |
|
Barium sulfate price More Price(33)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | NISTRM8557 | NBS 127 (sulfur and oxygen isotopes in barium sulfate) NIST? RM 8557 | 7727-43-7 | 0.5G | $826 | 2024-03-01 | Buy |
| Sigma-Aldrich | 243353 | Barium sulfate ReagentPlus , 99% | 7727-43-7 | 1kg | $220 | 2024-03-01 | Buy |
| Sigma-Aldrich | 1.01750 | Barium sulfate EMPROVE?ESSENTIALPhEur,BP,USP | 7727-43-7 | 5kg | $421 | 2024-03-01 | Buy |
| Sigma-Aldrich | 1.01750 | Barium sulfate EMPROVE?ESSENTIALPhEur,BP,USP | 7727-43-7 | 25kg | $1473 | 2024-03-01 | Buy |
| Sigma-Aldrich | 202762 | Barium sulfate 99.998% trace metals basis | 7727-43-7 | 20g | $150 | 2024-03-01 | Buy |
Barium sulfate Chemical Properties,Uses,Production
Chemical Properties
Barium sulfate has its chemical formula BaSO4. It is colorless or white orthorhombic crystals with relative molecular mass of 233.4, the relative density of 4.5 (15 ℃), the melting point of 1580 ℃, and the Refractive index of 1.637. Upon being heated to 1149 ℃, it will become monoclinic crystalline when the refractive index is 1.649. It is almost insoluble in water with the solubility being 0.00022 at 18 ℃ and 0.0041 at 100 ℃. It is slightly soluble in concentrated sulfuric acid and soluble in carbonate alkali metal solution in which it is converted to barium carbonate; it is insoluble in other kinds of acid or base. In nature, it is existed in the barite mineral form. Upon co-heating with carbon (pulverized coal) to 800 ℃, it is reduced to soluble barium sulfide and carbon monoxide. It has a strong ability for absorbing X-rays with X-ray being impermeable to it. Therefore, it is medically used as the agent (barium meal) for X-rays on the gut and stomach. Barium sulfate is the only non-toxic barium salts. It can be used in analysis reagents, electronics, instrumentation, metallurgy and other industries. It can also be used as a white pigment and an administrated agent upon stomach X-ray angiography; it can also used as copper flux, drilling mud weight enlarging agent and the filling agent of rubber, paper, and plastic. It is produced from the reaction between sulfuric acid and barium chloride.
I-type barium sulfate: it is white loose powder and is odorless and tasteless.
II-type barium sulfate: it is refined from barite sulfate type minerals: barium sulfate. Calculated from the dry goods, the barium sulfate content should not be less than 97%. It is white loose powder, and is odorless and tasteless. Barium sulfate (Ⅰ type and Ⅱ type) are both insoluble in water, organic solvent, acid o and sodium hydroxide. It contains 58.85% of barium.

Figure 1 is a white powder of barium sulfate.
The above information is edited by the Chemicalbook of Dai Xiongfeng.
Precautions
1. Patients of acute gastrointestinal perforation, gastrointestinal bleeding, colon infarction, acute gastroenteritis, and corrosive esophagitis should be disabled. Patients of congenital diseases such as tracheal fistula and esophageal atresia are not allowed for using this agent for examination. Instead, the patient can apply iodized oil or water-soluble contrast agent.
2. The barium sulfate must comply with the purity stated in Chinese Pharmacopeia without containing soluble barium salt. Avoid by all means mixing it with barium chloride (highly toxic) to avoid poisoning after absorption.
3. Fast for 6 to 12 hours before oral administration of barium agent; usually take this agent for examination during empty stomach in the morning. At 1 to 2 hours before barium enema, the patients must first subject to cleansing enema; the patients are not allowed for taking laxative at 1 day before the examination.
4. For patients with pyloric obstruction symptoms, apply gastric lavage for at least twice at the day before the test.
5. within 3 days before the examination, the patients are disabled for administering drugs with higher atomic weight such as bismuth, calcium preparations. At 1 day before the test, disable the using of drugs which affect stomach and intestine such as atropine, calcium, bismuth, laxatives, etc., it recommended to take low-residue food and fast after dinner.
6. The fineness and purity of barium sulfate powder should reach the standard comply of the codex. For self-production of the paste or suspension of this product, you can choose suitable dispersing agent and flavoring agent to prepare a suitable preparation with suitable consistency.
Chemical Properties
It is colorless orthorhombic crystals or a white amorphous powder. It is almost insoluble in water, ethanol and acid but soluble in hot concentrated sulfuric acid.
Uses
Barium sulfate (BaSO4), as mentioned, is used in medicine as an opaque liquid medium to block X-rays when ingested, thus providing an image of ulcers and intestinal problems. It is also used in the manufacture of paints, rubber, and plastics.
Production method
Mirabilite-black ash: mix the raw material of barium sulfide (refer to preparation of barium sulfide) with mirabilite which has calcium, magnesium being removed and carry out the reaction at 90 °C to generate the barium sulfate precipitate. The precipitate further undergoes filtration, washing with water and acid and adjusted with sulfuric acid to pH 5-6; again go through filtration, drying and pulverization to obtain the precipitated barium sulfate products. After addition of surface treatment agent or dispersant during the production process of precipitated barium sulfate, we can obtain modified superfine precipitated barium sulfate; the reaction is as below:
BaSO4 + 4C → BaS + 4CO ↑
BaS + Na2SO4 → BaSO4 ↓ + Na2S
Bittern utilization method applies yellow barium halide for reaction with mirabilite; then it further goes through acid cooking, washing with water, separation, dehydration drying to obtain the finished product of barium sulfate. The reaction is as below:
BaCl2 + Na2SO4 → BaSO4 ↓ + 2NaCl
Acute toxicity
the insoluble body portion has low toxicity; dissolved impurities is toxic; trachea-mouse LD50: > 600 mL/kg
Explosives and hazardous properties
heating together with aluminum, phosphorus causing explosion
Flammability and hazard characteristics
thermal decomposition releases toxic fumes of sulfur oxides
Storage Characteristics
ventilation, low-temperature and drying; stored separately from aluminum and potassium
Professional standards
TWA 0.5 mg/m³; STEL 1.5 mg/m3
Description
Barium sulfate is available as odourless, tasteless, white or yellowish crystals or powder or polymorphous crystals. It is stable and insoluble or negligibly soluble in water, and on burning, it may produce sulphur oxides. It reacts violently with aluminium powder. It occurs naturally as mineral barite, barytes. It has wide use as inert filler and pigment extender in paints, primers, inks, plastics, floor tiles, paper coatings, polymer fibres, and rubber. It is used as the semi-transparent base (lake) for organic pigments and as a thixotropic weighting mud in oil well drilling. Barium sulphate is a contrast agent that is used to help x-ray diagnosis of problems in areas of the upper GI tract, like the oesophagus, the stomach, and/ or the small intestine. The raw barium sulphate has wide applications such as in the manufacture of lithopone, a white pigment in the manufacture of photographic paper, wallpaper and glassmaking, in battery plate expanders, and in heavy concrete for radiation shield.
Chemical Properties
Barium sulfate is available as white powder or polymorphous crystals. It is stable, odor- less, insoluble, or negligibly soluble in water, and may produce sulfur oxides on burning. It occurs naturally as mineral barite (barytes). It has wide use as an inert i ller pigment extender in paints, primers, inks, plastics, l oor tiles, paper coatings, polymer i bers, and rubber. It is used as the semi-transparent base (lake) for organic pigments and as a thixo- tropic weighting mud in oil well drilling. Barium sulfate is radio-opaque and is used as bar- ium meal in medical x-ray diagnosis. Barium sulfate is a contrast agent that is used to help x-ray diagnosis of problems in areas of the upper GI tract, like the esophagus, the stomach, and/or the small intestine. Barium sulfate is the raw material for the manufacture of litho- pone, a white pigment, and is used in the manufacture of photographic paper, wallpaper, and glassmaking; in battery plate expanders; and in heavy concrete for radiation shield.
Physical properties
Barium sulfate has the molecular formula of BaSO4
and the molecular weight of 233.3896 g/mol. It can be
prepared by the reaction of barium carbonate and
sulfuric acid:
BaCO3+H2SO4?BaSO4+CO2+H2O
Barium sulfate is a soft crystalline solid. It is
a rhombic crystal. The pure salt is white but the color
of the mineral “barite” can vary between red, yellow,
gray or green, depending on impurities. Its density is
4.50 g/cm3 and its refractive index is 1.64. It melts
around 1580°C but decomposes above 1600°C. Its
hardness is 4.3 to 4.6 Mohs. It is virtually insoluble in
water (285 mg/l at 30°C) and insoluble in alcohol. Its
Ksp is 1.1×10-10. It is soluble in concentrated sulfuric
acid. The crystal structure of BaSO4 is known to be rhombic, with a space group pnma. The lattice parameters are: a=8.896? , b=5.462°, c=7.171 ?,
V=348.4 ?3.
Silica is the prime impurity that can be removed as
sodium fluorosilicate by treatment with hydrofluoric
acid followed by caustic soda. Very pure barium sulfate
may be obtained by treating an aqueous solution of
a soluble barium salt with sodium sulfate:
BaCl2+Na2SO4?BaSO4+2NaCl
Barium sulfate is one of the most insoluble salts of the
alkaline earths. It does not undergo double decomposition
reactions in aqueous phase like its Mg homologue.
It dissolves in concentrated H2SO4 to form an acid
sulfate that breaks down to BaSO4 upon dilution. Reduction
with coke under heating produces barium sulfide:
BaSO4+3C?BaS+2CO+CO2
Occurrence
Barium sulfate is widely distributed in nature and occurs as the mineral barite (also known as barytes or heavy spar). It often is associated with other metallic ores, such as fluorspar. Barites containing over 94% BaSO4 can be processed economically.
Barium sulfate has many commercial applications. It is used as natural barite, or precipitated BaSO4. The precipitated salt in combination with equimolar amount of co-precipitated zinc sulfide formerly was used as a white protective coating pigment, known as lithophone. Similarly, in combination with sodium sulfide, it is used to produce fine pigment particles of uniform size, known as blanc fixe. Natural barite, however, has greater commercial application than the precipitated salt. It is used as drilling mud in oil drilling to lubricate and cool the drilling bit, and to plaster the walls of the drill hole to prevent caving. It is used as a filler in automotive paints, plastics and rubber products. It also is used in polyurethane foam floor mats; white sidewall rubber tires; and as a flux and additive to glass to increase the refractive index.
Other chemical applications of barium sulfate are as the opaque ingredient in a barium meal for x-ray diagnosis; as a pigment for photographic paper; and to prepare many barium salts.
Uses
Barium sulfate has many commercial applications. It
is used either as natural barite, or precipitated BaSO4.
The precipitated salt in combination with equimolar
amount of co-precipitated zinc sulfide formerly was
used as a white protective coating pigment, known as
“lithopone”. Similarly, in combination with sodium
sulfide, it is used to produce fine pigment particles of
uniform size, known as “blanc fixe”. Natural barite,
however, has greater commercial application than the
precipitated salt. It is used as an additive in drilling
mud in crude oil, well drilled to lubricate and cool the
drilling bit, and to plaster the walls of the drill hole to
prevent caving. It is used as a filler in automotive paints,
plastics and rubber products. It also is used as a filler in
polyurethane foam floor mats, white sidewall rubber
tires and as a flux and additive to glass to increase the
refractive index.
Barium sulfate is frequently used clinically as
a contrast agent for X-ray imaging and other diagnostic
procedures. It is most often used in imaging of the
gastrointestinal tract. It is administered, orally or by
enema, as a suspension of fine particles in an aqueous
solution. Although barium, and its water-soluble
compounds are often highly toxic, the extremely low
solubility of barium sulfate protects the patient from
absorbing harmful amounts of the metal. Barium sulfate
is also readily removed from the body, unlike prior
compounds, which it replaced. Its absorbance of Xrays
is also higher.
Barium sulfate mixtures are used as white pigment
for paints. In oil paint, barium sulfate is almost transparent,
and is used as a filler or to modify consistency.
One major manufacturer of artists’ oil paint sells
“permanent white” that contains a mixture of titanium
white pigment and barium sulfate. Barium
sulfate itself is called blanc fixe (French for “permanent
white”).
range. It is applied by spray painting to almost any
substrate (metals, plastics, glass) for use in integrating
spheres, laser cavities, lamp reflectors and display backlights.
It is characterized by a near-perfect Lambertian
(i.e. diffuse) reflectance of up to 98% in the spectral
range from 250 to 2500 nm.
Other chemical applications of barium sulfate are
used as a pigment for photographic paper. It is also
used to prepare many other barium salts. It is available
in many forms commercially.
Uses
A heavy, white powder made by treating barium salts with sulfuric acid. Barium sulfate was used as a preliminary coating for raw photographic papers to produce a smooth, white surface and to act as a barrier to prevent reactions between the paper and subsequent coatings of gelatin or collodion emulsions. Barium sulfate was also added directly to emulsions to produce a matte finish. It is also known as barite, synthetic barite, blanc fix, baryta white, and mountain snow.
Uses
barium sulfate is an emulsion stabilizer for sunscreen formulations. outside of sunscreen preparations, this inorganic salt is most commonly used in non-cosmetic soaps.
Uses
Finepowder Barium Sulphate is widely used in chemical industrypaint, plastics, rubber,glass, paper, medicine,ceramics, storage battery and other areas;Fine powder Barium Sulphate is widely used in chemical industry
Definition
ChEBI: A metal sulfate with formula BaO4S. Virtually insoluble in water at room temperature, it is mostly used as a component in oil well drilling fluid it occurs naturally as the mineral barite.
Production Methods
Natural barium sulfate or barite is widely distributed in nature. It also contains silica, ferric oxide and fluoride impurities. Silica is the prime impurity which may be removed as sodium fluorosilicate by treatment with hydrofluoric acid followed by caustic soda.
Very pure barium sulfate may be precipitated by treating an aqueous solution of a barium salt with sodium sulfate:
BaCl2 + Na2SO4 → BaSO4 + 2NaCl
Precipitated BaSO4 is often used in many industrial applications. Blanc fixe and Lithopone are made by the reactions of barium sulfide with sodium sulfate and zinc sulfate, respectively.
brand name
Baricon (Mallinckrodt); Bar-test (Glenwood); Barocat (Mallinckrodt); Barosperse (Mallinckrodt); Barosperse II (Mallinckrodt); Barotrast (Rhone-Poulenc Rorer); Epi-C (Mallinckrodt); Epi-Stat 57 (Mallinckrodt); Epi-Stat 61 (Mallinckrodt); Esophotrast (Rhone-Poulenc Rorer); Oratrast (Rhone-Poulenc Rorer).
General Description
Barium sulphate is widely employed as an inorganic filler. Its crystals belong to the rhombic crystal system. The rhombic crystals transforms to monoclinic form at 1148°C and eventually it decomposes to barium oxide, sulfur dioxide and oxygen at temperatures above 1400°C.
Reactivity Profile
Barium sulfate is non-combustible and non-toxic. Emits toxic sulfur oxides when heated to decomposition. Can act as an oxidizing agent, but usually does not. Reacts with reducing agents such as potassium, phosphorus or aluminum (heating with aluminum can cause an explosion).
Hazard
Pneumoconiosis.
Health Hazard
Exposures to barium sulfate cause irritation to the eyes, lachrimation; redness, scaling, and itching are characteristics of skin inl ammation. Although barium sulfate has been identi- i ed as a non-toxic dust, long-term inhalation of dust in high concentrations has caused benign pneumoconiosis (baritosis), deposition of dust in the lungs in sufi cient quantities to produce adverse effects. This produces a radiological picture even though symptoms and abnormal signs may not be present. The Fumes of barium are respiratory irritants and over-exposure to dusts and fumes is known to cause rhinitis, frontal headache, wheezing, laryngeal spasm, salivation, and anorexia. Long-term effects include nervous disorders and adverse effects on the heart, circulatory system, and musculature. Heavy exposures to barium sulfate may cause benign pneumoconi in exposed workers. However, there are no reports indicating that barium sulfate has potential occupational hazards or carcinogenicity.
Pharmaceutical Applications
Barium salts can be highly toxic even at low concentrations. Barium carbonate is highly toxic and can be used as rat poison as it readily dissolves in the stomach acid. Barium sulfate is the least toxic barium compound mainly because of its insolubility. Barium sulfate is used in a variety of applications ranging from white paint to X-ray contrast agent. The clinical use of barium sulfate suspension is well known under the term barium meal. Patients are given a suspension of barium sulfate to swallow. Using X-ray imaging, the whole oesophagus, the stomach and the intestines can be visualised. Barium sulfate lines the tissue whilst travelling through the digestive tract. The heavy barium ions absorb X-rays readily and therefore these structures become visible in an X-ray screening. Barium sulfate is a well-used and tolerated oral radio-contrast agent. It is also used as radio-contrast agent in enemas.
Safety Profile
Questionable carcinogen with experimental tumorigenic data. Mutation data reported. A relatively insoluble salt used as an opaque medium in radtography. Soluble impurities can lead to toxic reactions. Heating with aluminum can produce an explosion. Incompatible with aluminum and potassium. When heated to decomposition it emits toxic fumes of SOx.
Potential Exposure
Barium sulfate is used as an opaque medium in radiography; as a mud weighting material in oil well drilling; in paper coating; as a paint pigment.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, rinsemouth and get medical attention immediately due to thepossibility of barium poisoning. See also “First Aid” sectionin “Barium” entry
storage
Barium sulfate should be kept stored in its original sealed glass container with proper security when not in use. Barium sulfate should be stored in a cool, dry, ventilated area, away from incompatible materials and foodstuff containers. The containers need to be protected against physical damage and checked regularly for leaks.
Shipping
UN1564 Barium compounds, n.o.s., Hazard Class: 6.1; Labels: 6.1—Poisonous materials.
Incompatibilities
May act as an oxidizer. Reacts with reducing agents such as hydrides, potassium, phosphorus or aluminum. Aluminum powder1heat may be violent; possibly explosive.
Precautions
Barium sulfate should only be used by or under the direct supervision of a qualii ed super- visor or doctor. During use and handling of barium sulfate, workers should avoid contact of the chemical substance with the skin and should not breathe dust. After use, the work- ers should wash their hands with soap and water. While dispensing, care is needed to label barium sulfate properly and correctly, to avoid confusion with poisonous barium suli de, suli te, or carbonate.
Barium sulfate Preparation Products And Raw materials
Raw materials
1of3
Preparation Products
1of6
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hebei Ningnan Trade Co. LTD | +86-18034019111 | admin@hbningnan.com | China | 278 | 58 |
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 | sales@hbmojin.com | China | 12456 | 58 |
| Hebei Yanxi Chemical Co., Ltd. | +8617531190177 | peter@yan-xi.com | China | 5993 | 58 |
| Henan Bao Enluo International TradeCo.,LTD | +86-17331933971 +86-17331933971 | deasea125996@gmail.com | China | 2503 | 58 |
| Hebei Saisier Technology Co., LTD | +86-18400010335 +86-13102810335 | admin@hbsaisier.cn | China | 690 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21691 | 55 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418679 +86-18949832763 | info@tnjchem.com | China | 2989 | 55 |
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Hebei Guanlang Biotechnology Co., Ltd. | +86-19930503282 | alice@crovellbio.com | China | 8823 | 58 |
Related articles
- Barium sulfate: Application, production and toxicity
- Barium sulfate (or sulphate) is the inorganic compound with the chemical formula BaSO4. It is a barium salt and a metal sulfat....
- May 17,2023
- Barium sulfate – a widely used Metal Sulfate
- Barium sulfate (or sulphate) is the inorganic compound with the chemical formula BaSO4. It is a barium salt and a metal sulfat....
- Nov 25,2019
View Lastest Price from Barium sulfate manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-04-24 | Barium sulfate
7727-43-7
|
US $5.00-3.50 / grams | 1grams | 99% | 100Tons | Hebei Dangtong Import and export Co LTD | |
 |
2024-04-23 | Barium sulfate
7727-43-7
|
US $6.00 / KG | 1KG | More than 99% | 2000KG/Month | Hebei Saisier Technology Co., LTD | |
 |
2023-12-25 | Barium sulfate
7727-43-7
|
US $0.00 / kg | 1kg | 98% | 1000000 | Hebei Jingbo New Material Technology Co., Ltd |
-

- Barium sulfate
7727-43-7
- US $5.00-3.50 / grams
- 99%
- Hebei Dangtong Import and export Co LTD
-

- Barium sulfate
7727-43-7
- US $6.00 / KG
- More than 99%
- Hebei Saisier Technology Co., LTD
-

- Barium sulfate
7727-43-7
- US $0.00 / kg
- 98%
- Hebei Jingbo New Material Technology Co., Ltd