メタン 化学特性,用途語,生産方法
解説
メタン,可燃性天然ガス,および有機物の腐敗,発酵により生成する沼気の主成分.石炭,石油系炭化水素などの熱分解により発生するガス中に含まれる.わが国では,おもに水素および合成ガスの原料,および都市ガスや火力発電所の燃料として用いられる.
森北出版「化学辞典(第2版)
反応
光のような刺激で励起されたハロゲン元素と、メタンは反応しやすいです。これは激しい発熱反応であり、水素原子からハロゲン原子へ置換されます。具体的に、常温でを含む混合気体を直射日光に当てると、メタンは発火します。
その一方で、1molのメタンは、完全燃焼によって1molと水2molになり、890kJの熱を得ることが可能です。メタンの不完全燃焼では、一酸化炭素と水が生じます。
性質
メタン,四面体構造をもつ.C-H0.1091 nm.無味,無臭,無色の引火性の気体.融点-182.48 ℃,沸点-161.49 ℃.水に難溶,エタノール,エーテル,炭化水素油に微溶.爆発範囲5.0~15.0体積%.
用途
アモルファスシリコン膜(太陽電池,感光ドラムなど)。
合成
工業的にメタンは、水素との反応によって、大量生産できます。実験室では、強塩基の存在下で酢酸塩を熱して脱炭酸させると生じます。例えば、とを加熱すると、メタンを得ることが可能です。室温で炭化アルミニウムを水で加水分解してもメタンは生成しますが、不純物による強烈な臭いがあります。
それ以外にも、メタン菌の嫌気醗酵でもメタンが生じます。ちなみに自然界で生じるほとんどのメタンは、メタン菌によって合成されており、この反応には強い嫌気度が必要です。
製法
実験室的には,ヨウ化メチルマグネシウム(グリニャール試薬)と水を反応させるか,または酢酸ナトリウムをソーダ灰とともに金属製フラスコ中で乾留する合成法がある.工業的には,天然ガス,石油系炭化水素分解ガス,コークス炉ガスなどから分離製造される.
説明
Methane is a colorless, odorless, flammable hydrocarbon gas that is the simplest alkane. The root word, met, in methane is derived from the Greek root word methe meaning wine. Methylene was used in the early 19th century as the name for methanol, which is wood alcohol, CH3OH. Methylene comes from methe + hydē, the latter being the Greek word for wood, so methylene would mean wine from wood. Methanol got the names methylene and wood alcohol because it was discovered by Robert Boyle (1627–1691) in the 17th century by the destruction distillation of wood. Destructive distillation involves heating in the absence of air.
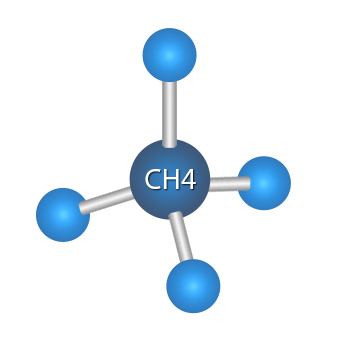
Methane is the first alkane and carries the suffix“ane” denoting an alkane, thus methe z + ane = methane. The carbon is at the center of the tetrahedron, which can be assumed to be an equilateral pyramid, with a hydrogen atom at each of the four corners of the tetrahedron.
Methane is the principal component of natural gas, with most sources containing at least 75% methane. Methane production occurs naturally through a process called methanogenesis. Methanogenesis involves anaerobic respiration by single-cell microbes collectively called methanogens.
化学的特性
Methane is a natural, colorless, odorless, and tasteless gas. It is used primarily as fuel to make heat and light. It is also used to manufacture organic chemicals. Methane can be formed by the decay of natural materials and is common in landfi lls, marshes, septic systems, and sewers. It is soluble in alcohol, ether, benzene, and organic solvents. Methane is incompatible with halogens, oxidizing materials, and combustible materials. Methane evaporates quickly. Methane gas is present in coal mines, marsh gas, and in sludge degradations. Methane can also be found in coal gas. Pockets of methane exist naturally underground. In homes, methane may be used to fuel a water heater, stove, and clothes dryer. Incomplete combustion of gas also produces carbon monoxide. Methane gas is flammable and may cause fl ash fi re. Methane forms an explosive mixture in air at levels as low as 5%. Electrostatic charges may be generated by fl ow and agitation.
来歴
Methane has been used as a fossilfuel for thousands of years. The discovery of methane
is attributed to the Italian physicist Alessandro Volta (1745–1827). Volta, known primarily for his discoveries in electricity, investigated reports of a flammable gas found in marshes. In
November 1776, Volta, while visiting the Lake Maggiore region of northern Italy, noticed that
gas bubbles emanated from disturbed sediments in marshes. Volta collected the gas and began
investigations on its nature. He discovered that the gas was highly flammable when mixed with
air. He developed an instrument termed Volta’s pistol (also called a spark eudiometer) that
fired metal balls like a miniature cannon to conduct combustion experiments with methane.
He also developed a lamp fueled by methane.
使用
Methane is used primarily as a fuel to make heat and light. It is also used to manufacture organic chemicals. Methane can be formed by the decay of natural materials and is common in landfills, marshes, septic systems, and sewers. It is soluble in alcohol, ether, benzene, and organic solvents. Methane is incompatible with halogens, oxidising materials, and combustible materials. Methane evaporates quickly. Methane gas is present in coal mines, marsh gas, and sludge degradations. Methane can also be found in coal gas. Pockets of methane exist naturally underground. In homes, methane may be used to fuel a water heater, stove, and clothes dryer. Also, incomplete combustion of gas also produces carbon monoxide. Methane gas is flammable and may cause flash fire. Methane forms an explosive mixture in air at levels as low as 5%. Electrostatic charges may be generated by flow and agitation.
定義
A gaseous
alkane. Natural gas is about 99% methane
and this provides an important starting
material for the organic-chemicals industry.
Methane can be chlorinated directly to
produce the more reactive chloromethanes,
or it can be ‘reformed’ by partial oxidation
or using steam to give mixtures of carbon
oxides and hydrogen. Methane is the first
member of the homologous series of alkanes.
調製方法
Methane is the end product of anaerobic decay. It is the
major constituent of natural gas, present at concentrations
between 600,000 and 800,000 ppm 60 to 80% of natural gas.
Methane collects in coal mines or geologically similar
earth deposit sites, evolves as marsh gas, and forms during
certain fermentation and sludge degradation processes.
Methane is also produced by decomposition in municipal
landfills; concentrations can be as high as 250,000 ppm.
It is often accompanied by other low molecular weight
hydrocarbons.
一般的な説明
METHANE is a colorless odorless gas. METHANE is also known as marsh gas or methyl hydride. METHANE is easily ignited. The vapors are lighter than air. Under prolonged exposure to fire or intense heat the containers may rupture violently and rocket. METHANE is used in making other chemicals and as a constituent of the fuel, natural gas.
空気と水の反応
Highly flammable.
反応プロフィール
METHANE is a reducing agent, METHANE is involved in many explosions when combined with especially powerful oxidizers such as bromine pentafluoride, chlorine trifluoride, chlorine, iodine, heptafluoride, dioxygenyl tetrafluoroborate, dioxygen difluoride, trioxygen difluoride and liquid oxygen. Other violent reactions include, chlorine dioxide and nitrogen trifluoride. Liquid oxygen gives an explosive mixture when combined with liquid METHANE [NFPA 1991]. Contact of very cold liquefied gas with water may result in vigorous or violent boiling of the product and extremely rapid vaporization due to the large temperature differences involved. If the water is hot, there is the possibility that a liquid "superheat" explosion may occur. Pressures may build to dangerous levels if liquid gas contacts water in a closed container [Handling Chemicals Safely 1980].
危険性
Severe fire and explosion hazard, forms
explosive mixture with air (5–15% by volume). An
asphyxiant gas.
健康ハザード
Methane is a relatively potent gas. It is the simplest alkane and the principal component of natural gas. Exposures to methane gas cause toxicity and adverse health effects. The signs and symptoms of toxicity include, but are not limited to, nausea, vomiting, diffi culty breathing, irregular heart beat, headache, drowsiness, fatigue, dizziness, disorientation, mood swings, tingling sensation, loss of coordination, suffocation, convulsions, unconsciousness, and coma. While at low concentrations methane causes no toxicity, high doses lead to asphyxiation in animals and humans. Displacement of air by methane gas is known to cause shortness of breath, unconsciousness, and death from hypoxemia. Methane gas does not pass readily through intact skin. However, in its extremely cold liquefi ed form, methane can cause burns to the skin and eyes. No long-term health effects are currently associated with exposure to methane.
火災危険
Special Hazards of Combustion Products: None
使用用途
メタンは都市ガスに使われている液化天然ガスの主成分であり、燃料用ガスとして使用されます。
メタンは、微生物によるメタン発酵により生ゴミ、家畜ふん尿を発酵させて得るバイオガスに含まれています。このバイオガスを精製してメタン濃度を90%以上にしたバイオメタンは、電力や熱として供給可能です。
さらに炭素を含む化合物を工業的に製造する際の原材料にもなります。例として、、、蟻酸はメタンを原材料として誘導されます。
农业用途
Biogas, a gaseous fuel, is produced by the fermentation
of organic matter by methane-forming bacteria
(methanogens). Biogas consists of a mixture of methane,
carbon dioxide and hydrogen.
A mixture of methane and carbon dioxide, or even
methane alone, formed in the deep layers of organic
material in swamp bottoms or landfills, is sometimes
called swamp gas or marsh gas.
Acetoclastic bacteria form methane exclusively
from acetic acid in anaerobic digestion. They grow
slowly and have a doubling time of several days, which
is the rate-limiting step in biogas production. Bacteria
that ferment fatty acids (mainly propionic acid and
butyric acid) to acetic acid are called acetogenic
bacteria.
Animal dung and plant residues are used to produce
biogas in a fermenter. The residual biogas slurry
containing 1.4 to 1.8 % nitrogen, 1.1 to 1.7 % phosphorus
(as P
2O
5)an d 0.8 to 1.3 % potassium (as K
2O) is used as
organic manure. Animal manure used for biogas
production does not lose its fertilizer nutrient value.
Biogas is usually made by the decomposition of
domestic, industrial and agricultural sewage wastes.
Methane, its major component, can be harvested and
used as a pollution-free renewable resource and a derived
source of domestic energy. Biogas, produced in special
biogas digesters, is widely used in China and India.
材料の用途
Methane is noncorrosive and may be contained
by any common, commercially available metals,
with the exception of cryogenic liquid applications.
Handling equipment must, however, be
designed to safely withstand the temperatures
and pressures to be encountered.
At the temperature of liquid methane, ordinary
carbon steels and most alloy steels lose their ductility
and are considered unsafe for liquid methane
service. Satisfactory materials for use with
liquid methane include Type 18-8 stainless steel
and other austenitic nickel-chromium alloys, copper,
Monel, brass, and aluminum.
安全性プロファイル
A simple asphyxiant.
Very dangerous fire and explosion hazard
when exposed to heat or flame. Reacts
violently with powerful oxidzers (e.g.,
bromine pentafluoride, chlorine trifluoride,
chlorine, fluorine, iodine heptafluoride,
dioxygenyl tetrafluoroborate, dioxygen
difluoride, trioxygen difluoride, liquid
oxygen, ClO2, NF3,OF2). Incompatible with
halogens or interhalogens in air (forms
explosive mixtures). Explosive in the form
of vapor when exposed to heat or flame. To
fight fire, stop flow of gas. See also
ARGON for a description of asphyxiants.
職業ばく露
Methane is used as a fuel and in the
manufacture of organic chemicals, acetylene, hydrogen
cyanide, and hydrogen. It may also be a cold liquid.
Natural gas is used principally as a heating fuel. It is transported as a liquid under pressure. It is also used in the manufacture of various chemicals including acetaldehyde,
acetylene, ammonia, carbon black; ethyl alcohol; formaldehyde, hydrocarbon fuels; hydrogenated oils; methyl alcohol; nitric acid; synthesis gas; and vinyl chloride. Helium
can be extracted from certain types of natural gas.
貯蔵
Occupational workers should store methane gas containers away from incompatible substances and handle in accordance with standard set regulations and grounding and bonding if required.
輸送方法
UN1971 Methane, compressed or Natural gas,
compressed (with high methane content), Hazard Class:
2.1; Labels: 2.1-Flammable gas. UN1972 Methane, refrigerated liquid (cryogenic liquid) or Natural gas, refrigerated
liquid (cryogenic liquid), with high methane content),
Hazard Class: 2.1; Labels: 2.1-Flammable gas. Cylinders
must be transported in a secure upright position, in a wellventilated truck. Protect cylinder and labels from physical
damage. The owner of the compressed gas cylinder is the
only entity allowed by federal law (49CFR) to transport
and refill them. It is a violation of transportation regulations to refill compressed gas cylinders without the express
written permission of the owner
純化方法
Dry methane by passing over CaCl2 and P2O5, then through a Dry-ice trap and fractionally distil it from a liquid-nitrogen trap. Oxygen can be removed by prior passage in a stream of hydrogen over reduced copper oxide at 500o, and higher hydrocarbons can be removed by chlorinating about 10% of the sample: the hydrocarbons, chlorides and HCl are readily separated from the methane by condensing the sample in the liquid-nitrogen trap and fractionally distilling it. Methane has also been washed with conc H2SO4, then solid NaOH and then 30% NaOH solution. It is dried with CaCl2, then P2O5, and condensed in a trap at liquid air temperature, then transferred to another trap cooled in liquid nitrogen. CO2, O2, N2 and higher hydrocarbons can be removed from methane by adsorption on charcoal. [Eiseman & Potter J Res Nat Bur Stand 58 213 1957, Beilstein 1 IV 3.] HIGHLY FLAMMABLE.
不和合性
May form explosive mixture with air.
A strong reducing agent. Incompatible with oxidizers
(chlorates, nitrates, peroxides, permanganates, perchlorates,
chlorine, bromine, fluorine, etc.); contact may cause fires
or explosions. Keep away from alkaline materials, strong
bases, strong acids, oxoacids, epoxides. Reacts violently
with bromine pentafluoride, chlorine dioxide, nitrogen trifluoride, oxygen difluoride and liquid oxygen. In general,
avoid contact with all oxidizers
廃棄物の処理
Return refillable compressed
gas cylinders to supplier. Incineration (flaring)
予防処置
Occupational workers should be careful during handling and management of methane gas because of its severe fi re and explosion hazard, particularly with pressurized containers. The containers may rupture or explode if exposed to suffi cient heat. Workers should avoid heat, flames, sparks, and other sources of ignition, and stop any leak if possible without personal risk. Workers should wear appropriate chemical-resistant gloves. Also, vapors should be reduced with water spray and keep unnecessary workers/people away from the place of chemical hazard. The closed spaces should be well ventilated before the workers enter. Methane is not toxic; however, it is highly flammable and may form explosive mixtures with air. Methane is violently reactive with oxidizers, halogens, and some halogen-containing compounds. Methane is also an asphyxiant and in enclosed areas displaces oxygen. Septic tanks, cesspools, and drywells present serious hazards, including septic cave-in or collapse, methane gas explosion hazards, and asphyxiation hazards. Occupational workers/work area supervisor should note the indications of methane gas poisoning: Soon after exposure to oxygen levels of less than 15% in air, if the workers feel symptoms of dizziness, headache, and tiredness, medical advice should be provided.
メタン 上流と下流の製品情報
原材料
準備製品