The definition and classification of coupling agent
Coupling agents refer to a class of additives being able to improve the property of the interface between polymer materials and fillers. It contains two kinds of groups of different properties presented in the molecular structure. One kind of group can have chemical reaction or have good compatibility with the polymer material. Another kind of group can form chemical bonds with the inorganic material or filler. It can improve the interfacial adhesion of the two materials and significantly improve the performance of filler or reinforce the polymer materials.
There are various kinds of coupling agents including silane coupling agent (trichloro vinyl silane, triethoxy vinyl silane, [gamma] aminopropyl-triethoxysilane, trichloropropene silane, etc.), titanate coupling agent (tetrabutyl titanate ester, tri-isostearyl titanate, isopropyl, tri-isopropyl titanate, di-iso-stearic acid ethyl phthalate, etc.), organic chromium coupling agent (methacrylic acid chromium complex), aluminate zirconium coupling agent and a polymer coupling agent.
It is mainly used in the polymer reinforcing material. It can be used as reinforcing agent for the surface treatment of the glass fiber. Silane coupling agent can also be used for polyolefin cross-linking or directly used for plastic, rubber blending modification or thickening.
Titanate coupling agent
Titanate coupling agent is a novel type of coupling agent developed by the Kenrich Petroleum Chemical Company (United state) in 1975. It has a unique structure with a good coupling efficacy on the thermoplastic polymer and the dry filler. According to the coupling mechanism between molecular structure and the filler surface, titanate coupling agent can be divided into four basic types: (1) single-alkoxy (2) single alkoxy group-pyrophosphate (3) chelated (4) ligand type.
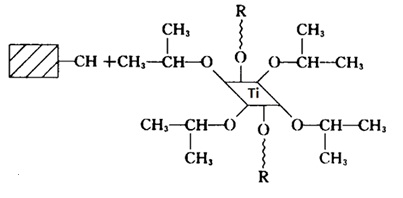
Figure 1 ligand-type titanate coupling agent
Titanate coupling agents should try to avoid being used in combination with additive of surface activity. They will interfere with the coupling reaction of titanate at the interface. In case these additives must be used, we should mix thoroughly fillers, coupling agents, and polymer before adding them.
Most titanate can have ester exchange reaction with ester-type plasticizer with varying degrees, therefore, the addition of ester plasticizer should be after the time when the fillers, coupling agents, conjugated polymer have been mixed thoroughly to form coupling agents.
Titanate coupling agent can be used in combination with silane coupling agent to produce a synergistic effect, for example use the chelated titanate for treatment of the glass fiber pre-treated with a silane coupling agent can greatly improve the coupling efficiency.
A silane coupling agent
Silane coupling agent is the earliest studied as well as most widely used coupling agent. It was developed by the US Company Union Carbide (U.C.C.) for the development of glass fiber reinforced plastic with more than 30 years of history.
It is organosilicon monomer having two or more different reactive groups in the molecule structure and can having chemically bonding (coupling) with organic material and inorganic material. The chemical formula of the silane coupling agent is: RSiX3. X represents a hydrolyzable functional group, and it may have coupling reaction with methoxy, ethoxy, cellosolve and inorganic materials (glass, metal, of SiO2). R represents an organic functional group; it can have coupling reaction with vinyl group, ethoxy group, methacrylic group, amino group, mercapto group and organic group as well as inorganic materials, various kinds of synthetic resins and rubber.
Name MW Relative density (25°C) Refractive index (25°C) Flash point/(25°C) Boiling point/(25°C, 101.324X103Pa)
Trichlorovinylsilane 161.5 1.26 1.432 21 19
Vinyltriethoxysilane 190.3 0.93 1.395 54 161
Vinyltri(β-methoxy-ethoxy)silane 280.4 1.04 1.428 66 285
(γ-glycidoxypropyl)trimethoxysilane 236.1 1.07 1.427 135 290
γ-methyl acrylyl ethyl trimethoxy silane 248.1 1.04 1.429 138 255
N-(β-aminoethyl)-γ- Aminopropyl-trimethoxy silane 222.1 1.03 1.445 140 259
N-(β-aminoethyl)-γ- Aminopropyl-methyl-trimethoxy silane 206.1 0.98 1.445 140 234
γ- chloropropyl -trimethoxy silane 198.5 1.08 1.418 78 192
γ-mercaptopropyl -trimethoxy silane 196.1 1.06 1.439 102 212
γ- Aminopropyl-trimethoxy silane 221 0.94 1.419 104 217
the physicochemical properties of several typical commonly used silanes coupling agent.
According to the reaction mechanism of the silane coupling agent, the hydrolyzable functional group X will generate silanol after coming across water. For an inorganic material (e.g. glass), the coupling agent will have condensation reaction with the silanol on the glass surface, forming a covalent bond between the glass and the silane coupling agent. Taking advantage of this feature, the silane coupling agent can be used to process glass fiber (manufacturing of reinforced plastics), improve the performance of coatings and adhesives as well as processing the surface of inorganic fillers, etc. For glass fiber reinforced unsaturated polyester, it is suitable to choose methacryloxy silanes; for epoxy laminates, it is preferably to use epoxidized silane and aminosilane. The new application of the silane coupling agent is being taken as a cross-linking agent of polyethylene, have copolymerization through the connection of polyethylene and vinyltrimethoxysilane, or by cross-linking through the condensation reaction between polyethylene and the silane.
The polyethylene after treatment can be used as cables and complex heterotype materials. In order to adapt to the development of functional polymer composites, it have been developed of some new silane coupling agents such as γ- ureido propyl-trimethoxysilane, γ- glycidyl propyl-methyl- diethoxysilane aminopropyl and N- phenyl -γ-dimethoxysilane and so on.
Application of coupling agent in glass fiber reinforced polyester resin
A key technology in the glass fiber reinforced polyester resin is to solve problems related to the binding between glass fiber and resin interface. This binding may be either physical bonding or chemical bonding. However, only chemical bonding can provide the strongest chemical bonding between the glass fibers and the resin. Taking advantage of coupling agents can achieve the chemical bonding.
There are majorly two types of coupling agents: one is an organic silicon compound; the other is an organic chromium complex.
The general formula of the silicone coupling agent is R4-nSiXn, wherein R is an organic group, X is OR ', Cl, R "and other easily hydrolyzable group, R', R" is a hydrocarbon group, n = 1, 2, 3, most of the n = 3. The organic group R can have chemical reaction with the unsaturated polyester with the easily hydrolyzed groups being subject to hydrolysis and condensation. When it have interaction with glass surface, it may have the following results:
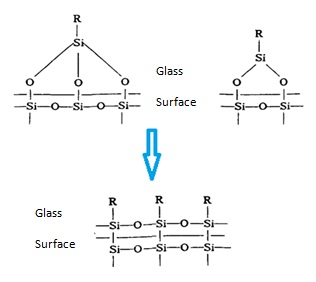
Figure2 the result of the interaction between an organic silane coupling agent and the glass surface
The result of the interaction between the coupling agent and the glass surface is generation of a strong covalent bond, thus changing the original properties of the glass surface, making it have hydrophobic, organophilic and oleophilic properties, thereby improving the mechanical properties of the composites.
Organic chromium complex coupling agents mainly contain methacrylate chromic chloride, being able to be applied to unsaturated polyester resins to improve the binding condition between glass fiber and resin interface. Methacrylic acid, chromium chloride, can bind to the glass surface through its hydroxyl group and the silanol surface contained after hydrolysis, and further outwardly projecting the methacrylic group to facilitate its chemically bonding to the resin; the process is as follows:
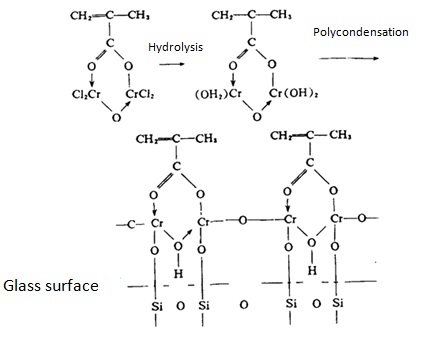
Figure 3 the result of the interaction between organic chromium coupling agent with the glass surface