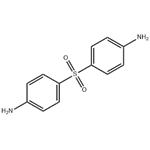
- 4,4'-Diaminodiphenylsulfone
-
- $100.00 / 1KG
-
2023-12-24
- CAS:80-08-0
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: g-kg-tons, free sample is available
- 4-Aminophenyl sulfone
-

- $0.00 / 25KG
-
2023-09-08
- CAS:80-08-0
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 50000KG/month
Related articles - Side effects of Dapsone
- Dapsone (4,4'-diaminodiphenylsulfone) is a sulfone that was the first effective antimicrobial for the treatment of leprosy. It....
- Mar 9,2022
|
| | 4,4'-Diaminodiphenylsulfone Basic information |
| | 4,4'-Diaminodiphenylsulfone Chemical Properties |
| Melting point | 175-177 °C(lit.) | | Boiling point | 511.7±35.0 °C(Predicted) | | density | 1.2701 (rough estimate) | | vapor pressure | 0.004Pa at 25℃ | | refractive index | 1.5950 (estimate) | | storage temp. | 2-8°C | | solubility | 0.38g/l | | form | Crystalline Powder | | pka | pKb 13.0(at 25℃) | | color | White to beige | | PH | 5.5-7.5 (H2O, 20℃)(saturated aqueous solution) | | Water Solubility | <0.1 g/100 mL at 20 ºC | | Merck | 14,2822 | | BRN | 788055 | | BCS Class | 4,2 | | Stability: | Stable. Combustible. Incompatible with strong oxidizing agents. | | InChIKey | MQJKPEGWNLWLTK-UHFFFAOYSA-N | | LogP | 0.97 at 25℃ | | CAS DataBase Reference | 80-08-0(CAS DataBase Reference) | | NIST Chemistry Reference | Dapsone(80-08-0) | | IARC | 3 (Vol. 24, Sup 7) 1987 | | EPA Substance Registry System | Dapsone (80-08-0) |
| Hazard Codes | Xn | | Risk Statements | 22 | | Safety Statements | 22 | | RIDADR | 3249 | | WGK Germany | 1 | | RTECS | BY8925000 | | TSCA | Yes | | HazardClass | 6.1(b) | | PackingGroup | III | | HS Code | 29051620 | | HS Code | 29309070 | | Hazardous Substances Data | 80-08-0(Hazardous Substances Data) | | Toxicity | LD50 orally in Rabbit: 1000 mg/kg LD50 dermal Rabbit > 4000 mg/kg |
| | 4,4'-Diaminodiphenylsulfone Usage And Synthesis |
| Chemical Properties | Off -White Crystalline Solid | | Originator | Avlosulfon,Ayerst,US,1957 | | Uses | Hardening agent in the curing of epoxy resins. | | Uses | antibacterial, leprostatic, dermatitis herpetiformis suppressant | | Uses | An antibacterial used in the treatment of dermatitis herpetiformis | | Uses | 4,4'-diaminodiphenylsulfone be used for preparation polyimide and epoxy resin material. | | Definition | ChEBI: A sulfone that is diphenylsulfone in which the hydrogen atom at the 4 position of each of the phenyl groups is substituted by an amino group. It is active against a wide range of bacteria, but is mainly employed for its actions against Mycobacteriu
leprae, being used as part of multidrug regimens in the treatment of all forms of leprosy. | | Indications | Although dapsone (Avlosulfon) was once used in the
treatment and prophylaxis of chloroquine-resistant P.
falciparum malaria, the toxicities associated with its
administration (e.g., agranulocytosis, methemoglobinemia,
hemolytic anemia) have severely reduced its use.
Occasionally dapsone has been added to the usual
chloroquine therapeutic regimen for the prophylaxis of
chloroquine-resistant P. falciparum malaria. It is also
used in combination therapy for leprosy. | | Indications | Although dapsone (Avlosulfon) is most often used as an
antimicrobial agent, it has important antiinflammatory
properties in many noninfectious skin diseases. The mechanism of action of dapsone in skin disease is
not clear.Most of the cutaneous diseases for which it is
effective manifest inflammation and are characterized
by an infiltration of neutrophils; the drug’s antiinflammatory
effect may arise from its inhibition of intracellular
neutrophil reactions mediated by myeloperoxidase
and hydrogen peroxide or from its scavenging of reactive
oxygen species, which inhibits inflammation. | | Manufacturing Process | p-Chloronitrobenzene is reacted with NaSO2C6H5NHCOCH3 to give as an
intermediate, O2NC6H5SO2C6H5NHCOCH3 which is then reduced and
deacetylated to give the product, dapsone. Alternatively, benzene and sulfuric
acid react to give phenyl sulfone which is nitrated, then reduced to give
dapsone. | | Brand name | Hansolar (ParkeDavis). | | Therapeutic Function | Antibacterial (leprostatic) | | Synthesis Reference(s) | Synthesis, p. 640, 1981 DOI: 10.1055/s-1981-29557 | | Antimicrobial activity | Dapsone is active against many bacteria and some protozoa.
Fully susceptible strains of M. leprae are inhibited by a little
as 0.003 mg/L. It is predominantly bacteristatic. Resistance
is associated with mutations in the folP1 gene involved in the
synthesis of para-aminobenzoic acid. | | Acquired resistance | Resistance to high levels is acquired by several sequential mutations.
As a result of prolonged use of dapsone monotherapy,
acquired resistance emerged in patients with multibacillary leprosy
in many countries. Initial resistance also occurs in patients
with both paucibacillary and multibacillary leprosy. Thus,
leprosy should always be treated with multidrug regimens.
Resistance of M. leprae to dapsone (and other anti-leprosy
drugs) may now be determined by use of DNA microarrays. | | General Description | Odorless white or creamy white crystalline powder. Slightly bitter taste. | | Air & Water Reactions | Sensitive to oxidation and light. Insoluble in water. | | Reactivity Profile | 4,4'-Diaminodiphenylsulfone can neutralize acids in exothermic reactions to form salts plus water. May be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen is generated in combination with strong reducing agents, such as hydrides. Incompatible with strong oxidizing agents. Also incompatible with epoxy resins under uncontrolled conditions . | | Fire Hazard | 4,4'-Diaminodiphenylsulfone is probably combustible. | | Pharmaceutical Applications | The most effective of a number of sulfonamide derivatives to
be tested against leprosy. The dry powder is very stable. It is
only slightly soluble in water. | | Pharmacokinetics | Oral absorption: >90%
Cmax 100 mg oral: c. 2 mg/L after 3–6 h
Plasma half-life: 10–50 h
Plasma protein binding: c. 50%
It is slowly but almost completely absorbed from the intestine
and widely distributed in the tissues, but selectively
retained in skin, muscle, kidneys and liver. It is metabolized
by N-oxidation and also by acetylation, which is subject to the
same genetic polymorphism as isoniazid. The elimination
half-life is consequently very variable, but on standard
therapy the trough levels are always well in excess of inhibitory
concentrations. It is mostly excreted in the urine: in the
unchanged form (20%), as N-oxidation products (30%) and
as a range of other metabolites. | | Clinical Use | Leprosy (multidrug regimens)
Prophylaxis of malaria, treatment of chloroquine-resistant malaria (in
combination with pyrimethamine)
Prophylaxis of toxoplasmosis (in combination with pyrimethamine)
Prophylaxis (monotherapy) and treatment (in combination with
trimethoprim) of Pneumocystis jirovecii pneumonia
Dermatitis herpetiformis and related skin disorders | | Clinical Use | Dapsone (4,4 -sulfonylbisbenzeneamine; 4,4 -sulfonyldianiline;p,p -diaminodiphenylsulfone; or DDS [Avlosulfon])occurs as an odorless, white crystalline powder that is veryslightly soluble in water and sparingly soluble in alcohol.The pure compound is light stable, but traces of impurities,including water, make it photosensitive and thus susceptibleto discoloration in light. Although no chemical change is detectablefollowing discoloration, the drug should be protectedfrom light.
Dapsone is used in the treatment of both lepromatous andtuberculoid types of leprosy. Dapsone is used widely for allforms of leprosy, often in combination with clofazimine andrifampin. Initial treatment often includes rifampin with dapsone,followed by dapsone alone. It is also used to preventthe occurrence of multibacillary leprosy when given prophylactically.Dapsone is also the drug of choice for dermatitis herpetiformisand is sometimes used with pyrimethamine for treatmentof malaria and with trimethoprim for PCP.
Serious side effects can include hemolytic anemia,methemoglobinemia, and toxic hepatic effects. Hemolyticeffects can be pronounced in patients with glucose-6-phosphatedehydrogenase deficiency. During therapy, all patientsrequire frequent blood counts. | | Clinical Use | Dapsone is approved for the treatment of an autoimmune
blistering skin disease, dermatitis herpetiformis.
This intensely pruritic eruption is characterized
histologically by a dense dermal infiltration of neutrophils
and subepidermal blisters. Other skin diseases
in which dapsone is helpful are linear immunoglobulin
A (IgA) dermatosis, subcorneal pustular dermatosis,
leukocytoclastic vasculitis, and a variety of rarer eruptions
in which neutrophils predominate, including some
forms of cutaneous lupus erythematosus. | | Side effects | Although usually well tolerated at standard doses, gastrointestinal
upsets, anorexia, headaches, dizziness and insomnia may
occur. Less frequent reactions include skin rashes, exfoliative
dermatitis, photosensitivity, peripheral neuropathy (usually
in non-leprosy patients), tinnitus, blurred vision, psychoses,
hepatitis, nephrotic syndrome, systemic lupus erythematosus
and generalized lymphadenopathy.
The term ‘dapsone syndrome’ is applied to a skin rash and
fever occurring 2–8 weeks after starting therapy and sometimes
accompanied by lymphadenopathy, hepatomegaly,
jaundice and/or mononucleosis.
Blood disorders include anemia, methemoglobinemia,
sulfhemoglobinemia, hemolysis (notably in patients with
glucose-
6-phosphate dehydrogenase deficiency), mononucleosis,
leukopenia and, rarely, agranulocytosis. Severe anemia
should be treated before patients receive dapsone.
The incidence of adverse reactions declined in the 1960s
but reappeared around 1982 when multidrug therapy was
introduced, and may represent an unexplained interaction
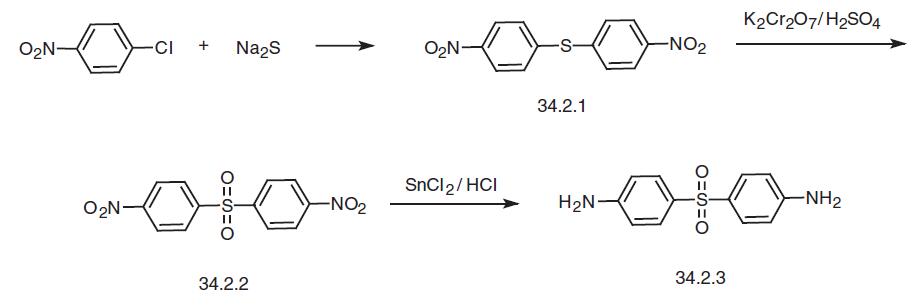
with rifampicin. | | Safety Profile | Poison by ingestion, intraperitoneal, and subcutaneous routes. Human systemic effects by ingestion: agranulocytosis, change in tubules and other kidney changes, cyanosis, effect on joints, hemolysis with or without anemia, jaundice, methemoglobinemiacarboxyhemoglobinemia, retinal changes, somnolence. Experimental reproductive effects. Can cause hepatitis, dermatitis, and neuritis. Questionable carcinogen with experimental carcinogenic and neoplastigenic data. Human mutation data reported. Used in leprosy treatment and veterinary medicine. When heated to decomposition it emits very toxic fumes of NOx and SOx. See also SULFONATES. | | Synthesis | Dapsone, 4,4�-diaminodiphenylsulfone (34.2.3), is synthesized from either 4-chloronitrobenzene or from the sodium salt of 4-acetamidobenzenesulfonic acid. Reacting 4-chloronitrobenzene with sodium sulfide gives 4,4�-dinitrodiphenylthioester (34.2.1), and oxidation of the sulfur atom in this compound using potassium dichromate in sulfuric acid gives 4,4�-dinitrodiphenylsulfone (34.2.2). Reduction of the nitro group in the resulting compound using tin dichloride in hydrochloric acid makes the desired dapsone.
It has also been suggested to reduce the nitro group to an amino group, protect it with an acetyl protection, oxidize the sulfur atom to a sulfone using potassium dichromate, and then remove the protective acetyl group by hydrolysis.
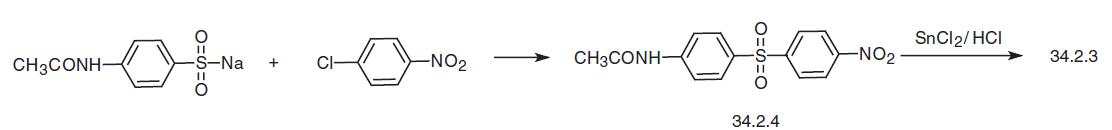
Another way of the synthesis of dapsone begins with 4-acetamidobenzenesulfonic acid, which is reacted with 4-chloronitrobenzene at high temperatures to give 4-acetamido-4�- nitrodiphenylsulfone (34.2.4). Reducing the nitro group in this compound with tin dichloride in hydrochloric acid along with the simultaneous hydrolysis of the acetyl group under the reaction conditions gives the desired dapsone.
 | | Drug interactions | Potentially hazardous interactions with other drugs
Antivirals: increased risk of ventricular arrhythmias
with saquinavir - avoid. | | Metabolism | Dapsone undergoes enterohepatic recycling. Dapsone is
acetylated to monoacetyldapsone, the major metabolite,
and other mono and diacetyl derivatives. Acetylation
shows genetic polymorphism. Hydroxylation is the other
major metabolic pathway resulting in hydroxylamine
dapsone, which may be responsible for dapsone�associated methaemoglobinaemia and haemolysis.
Dapsone is mainly excreted in the urine, only 20% of a
dose as unchanged drug. |
| | 4,4'-Diaminodiphenylsulfone Preparation Products And Raw materials |
|