- Pipracil
-
- $0.00 / 1kg
-
2023-11-01
- CAS:61477-96-1
- Min. Order: 1kg
- Purity: 99.0%
- Supply Ability: 20 tons
- Piperacillin
-

- $0.00 / 25KG
-
2023-07-01
- CAS:61477-96-1
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 50000KG/month
- Piperacillin
-

- $200.00 / 1kg
-
2023-06-26
- CAS:61477-96-1
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 1000kg/Month
|
| | Piperacillin Basic information |
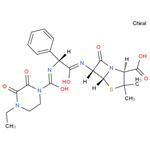
| Product Name: | Piperacillin | | Synonyms: | PIPERACILLIN EPP(CRM STANDARD);PIPERACILLIN USP(CRM STANDARD);PIPERAILLIN;4-Thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid, 6-[[(2R)-[[(4-ethyl-2,3-dioxo-1-piperazinyl)carbonyl]amino]phenylacetyl]amino]-3,3-dimethyl-7-oxo-, (2S,5R,6R)- (9CI);4-Thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid, 6-[[[[(4-ethyl-2,3-dioxo-1-piperazinyl)carbonyl]amino]phenylacetyl]amino]-3,3-dimethyl-7-oxo-, [2S-[2a,5a,6b(S*)]]-;Piperacillin (base and/or unspecified salts);[2S-[2alpha,5alpha,6beta(S*)]]-6-[[[[(4-ethyl-2,3-dioxopiperazin-1-yl)carbonyl]amino]phenylacetyl]amino]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid;Pipracil | | CAS: | 61477-96-1 | | MF: | C23H27N5O7S | | MW: | 517.55 | | EINECS: | 262-811-8 | | Product Categories: | Aromatics;Heterocycles;Intermediates & Fine Chemicals;Pharmaceuticals;Sulfur & Selenium Compounds;Antibiotic Explorer;61477-96-1 | | Mol File: | 61477-96-1.mol | 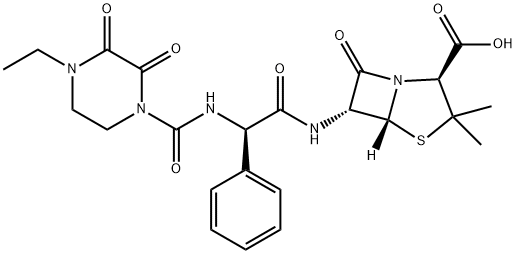 |
| | Piperacillin Chemical Properties |
| Melting point | 183-185?C (dec.) | | density | 1.51±0.1 g/cm3(Predicted) | | vapor pressure | 0Pa at 20℃ | | RTECS | XH8952200 | | storage temp. | Sealed in dry,2-8°C | | solubility | Freely soluble in methanol. Only sparingly soluble in aqueous solution at 0.119 mg/mL | | pka | 2.44±0.50(Predicted) | | form | Solid | | color | White to Off-White | | Water Solubility | 256.8mg/L at 20℃ | | Stability: | Hygroscopic | | LogP | -1.55 at 20℃ | | CAS DataBase Reference | 61477-96-1(CAS DataBase Reference) |
| | Piperacillin Usage And Synthesis |
| Chemical Properties | White Crystalline Solid; odorless; slightly hygroscopic. It is easily soluble in methanol, soluble in absolute ethanol or acetone, and very slightly soluble in water. It is a semi-synthetic penicillin antibiotic with broad-spectrum antibacterial effect. | | Originator | Pentcillin, Toyama ,Japan ,1980 | | Uses | Piperacillin is a semisynthetic penicillin with wide spectrum of antimicrobial activity, particularly pseudomonas strains. It is used to treat moderate-to-severe infections due to susceptible organisms. | | Definition | ChEBI: Piperacillin is a penicillin in which the substituent at position 6 of the penam ring is a 2-[(4-ethyl-2,3-dioxopiperazin-1-yl)carboxamido]-2-phenylacetamido group. It has a role as an antibacterial drug. It is a penicillin and a penicillin allergen. It is a conjugate acid of a piperacillin(1-). | | Brand name | Pipracil (Wyeth). | | Therapeutic Function | Antibiotic | | Antimicrobial activity | It displays good activity against non-β-lactamaseproducing
strains of N. gonorrhoeae, ampicillin-susceptible
H. influenzae and many Enterobacteriaceae. It is the most
active of the antipseudomonal penicillins against Ps. aeruginosa
and retains its activity in the absence of a β-lactamase
inhibitor. Synergy with aminoglycosides has been demonstrated
against many strains of Enterobacteriaceae and Ps.
aeruginosa. | | Acquired resistance | There is complete cross-resistance with other ureidopenicillins,
but ticarcillin-resistant strains of Ps. aeruginosa may
be susceptible. Piperacillin-resistant strains of B. fragilis
and other Bacteroides spp. are common. Because piperacillin
is hydrolyzed by most β-lactamases, many β-lactamaseproducing
isolates are resistant unless it is protected by
β-lactamase inhibitors. | | Pharmacokinetics | Oral absorption: Negligible
Cmax 2 g (2–3 min intravenous injection): 305 mg/L after 5 min
Plasma half-life: 0.9 h
Volume of distribution: 16–24 L/1.73 m2
Plasma protein binding: 16%
In patients with meningitis, mean CSF penetration of 30%
has been found. The urine is the principal route of excretion,
50–70% of the dose appearing over 12 h, most in the first
4 h. Most is excreted via the tubules, 75–90% in active form.
The half-life is prolonged in renal failure but much less than
is the case with carboxypenicillins. There is substantial biliary
excretion, levels in the common duct bile after a 1 g intravenous
dose commonly reaching 500 mg/L or more. During
hemodialysis the plasma half-life remains elevated and only
10–15% of the dose is removed. | | Clinical Use | Piperacillin (Pipracil) is the most generally useful of the extended-spectrum acylureidopenicillins. It is more active thanmezlocillin against susceptible strains of Gram-negativeaerobic bacilli, such as Serratia marcescens, Proteus,Enterobacter, Citrobacter spp., and P. aeruginosa.Mezlocillin, however, appears to be more active againstProvidencia spp. and K. pneumoniae. Piperacillin is alsoactive against anaerobic bacteria, especially B. fragilis andS. faecalis (enterococcus). β-Lactamase–producing strainsof these organisms are, however, resistant to piperacillin,which is hydrolyzed by S. aureus β-lactamase. The β-lactamase susceptibility of piperacillin is not absolute becauseβ-lactamase–producing, ampicillin-resistant strainsof N. gonorrhoeae and H. influenzae are susceptible topiperacillin.
Piperacillin is destroyed rapidly by stomach acid; therefore,it is active only by intramuscular or intravenousadministration. The injectable form is provided as the white,crystalline, water-soluble sodium salt. Its pharmacokineticproperties are very similar to those of the other acylureidopenicillins. | | Side effects | Piperacillin is generally well tolerated, with mild to moderate
pain on injection, thrombophlebitis and diarrhea in some
patients. It otherwise exhibits side effects common to the
group, including hypersensitivity, leukopenia and abnormalities
of platelet aggregation without coagulation defect, except
on prolonged treatment. | | Synthesis | Piperacillin, (2S,5R,6R)-3,3-dimethyl-7-oxo-6-[(2R)-2-[(4-ethyl-2,3-dioxo-
1-piperazinyl)formamido]-2-phenylacetamido]-4-thia-1-azabicyclo[3.2.0]-heptan-2-car�boxylic acid (32.1.1.30), is also synthesized by acylating ampicillin (32.1.1.16), but with
1-chlorocarbonyl-4-ethylpiperazin-2,3-dione (32.1.1.29). The necessary 1-chlorocarbonyl-4-
ethylpiperazin-2,3-dione (32.1.1.29) is synthesized by reacting N-ethylethylenediamine with
diethyloxalate, forming 4-ethylpiperazin-2,3-dione (32.1.1.28), and then acylating this with
phosgene after initial silylation of the product at the nitrogen atom with trimethylchlorosilane. 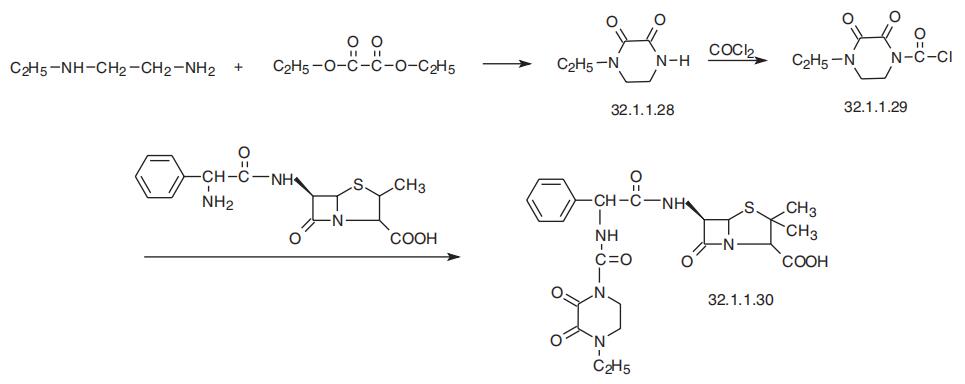
| | Mode of action | Piperacillin binds to penicillin binding proteins (PBP) located on the inner membrane of the bacterial cell wall, thereby interfering with the cross-linking of peptidoglycan chains necessary for bacterial cell wall strength and rigidity. As a result, cell wall synthesis is interrupted leading to a weakened cell wall and eventually cell lysis. |
| | Piperacillin Preparation Products And Raw materials |
|