|
|
| | VERRUCULOGEN Basic information |
| Product Name: | VERRUCULOGEN | | Synonyms: | VERRUCULOGEN;VERRUCULOGEN, PENICILLIUM VERRUCULOSUM;3h)-dione,1,10,10a,14,14a,15b-hexahydro-10,10a-dihydroxy-7-methoxy-2,2-dimeth;5h,12h-3,4-dioxa-5a,11a,15a-triazacyclooct(lm)indeno(5,6-b)fluorene-11,15(2h,1;tr1;verruculogentr1;yl-5-(2-methyl-1-propenyl)-,(5r-(5-alpha,10-alpha,10a-alpha,14a-alpha-15b-alp;verruculogen from penicillium*verruculosum | | CAS: | 12771-72-1 | | MF: | C27H33N3O7 | | MW: | 511.57 | | EINECS: | 634-189-1 | | Product Categories: | antibiotic | | Mol File: | 12771-72-1.mol | 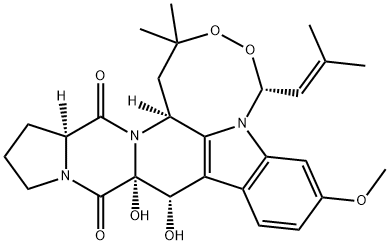 |
| | VERRUCULOGEN Chemical Properties |
| Boiling point | 738.4±60.0 °C(Predicted) | | density | 1.49±0.1 g/cm3(Predicted) | | Fp | 2℃ | | storage temp. | 2-8°C | | solubility | Soluble in ethanol;Soluble in methanol;Soluble in DMSO;Soluble in dimethyl formamide | | form | buffered aqueous solution | | pka | 10?+-.0.70(Predicted) | | color | White to off-white |
| | VERRUCULOGEN Usage And Synthesis |
| Uses | Verruculogen is a tremorgenic mycotoxin, first isolated from Penicillium verruculosum in 1972. The structure was resolved as an indole alkaloid in 1974. Verruculogen is produced by several species of Penicillium and Aspergillus and its presence is a useful taxonomic phenotypic marker. The tremorgenic action of verruculogen is associated with increases in spontaneous glutamate and aspartate release, decreases in GABA levels and, at toxic doses, an increase in the number and decrease in the affinity of DHP receptors in rat cortex. In in vitro guinea pig ileum preparations, verruculogen causes an increase in contractile responses due to electrical field stimulation, attributed to enhancement of acetylcholine from presynaptic nerve terminals. Verruculogen also inhibits Ca2+-activated K+ channels, and is a cell cycle inhibitor blocking division at the M phase. | | Uses | Suggested starting dilutions are as follows: ICC/IF: 1:100-1:1000, IHC-P: 1:100-1:1000, WB: 1:500-1:3000. Not yet tested in other applications. Optimal working dilutions should be determined experimentally by the end user. | | Uses | Verruculogen is a tremorgenic mycotoxin isolated from species of Penicillium, Aspergillus, and other fungi. It selectively inhibits the activation of Maxi-K potassium channels by charybdotoxin with a K1/2 value of 170 nM. Verruculogen also promotes the release of excitatory neurotransmitters when injected directly into the brain of rats and, at high doses, arrests mouse mammary carcinoma cells in M phase of the cell cycle (MIC = 12.2 μM).[Cayman Chemical] | | Definition | ChEBI: An organic heterohexacyclic compound that is a mycotoxic indole alkaloid isolated from Penicillium and Aspergillus species. | | Biological Activity | verruculogen is a maxi-k potassium channels inhibitor.maxi-k, a potassium channel characterized by their large conductance for potassium ions through cell membranes, is activated by changes in membrane electrical potential and/or by increases in concentration of intracellular calcium ion. maxi-k channels are critical for the regulation of several key physiological processes, such as smooth muscle tone and neuronal excitability. | | Biochem/physiol Actions | The protein encoded by this gene is a G protein-coupled receptor and is a component of the heterodimeric amino acid taste receptor T1R1+3. The T1R1+3 receptor responds to L-amino acids but not to D-enantiomers or other compounds. Most amino acids that are perceived as sweet activate T1R1+3, and this activation is strictly dependent on an intact T1R1+3 heterodimer. Multiple transcript variants encoding several different isoforms have been found for this gene. [provided by RefSeq] | | in vitro | in a previous study, it was found that aflatrem, paspalitrem a, paspalitrem c, penitrem a, and paspalinine could inhibit the binding of [125i]charybdotoxin to maxi-k channels in bovine aortic smooth muscle sarcolemmal membranes. whereas, verruculogen enhanced toxin binding. despite their different effects on binding of [125i]charybdotoxin to maxi-k channels, all tested compounds including verruculogen were able to potently inhibit maxi-k channels in electrophysiological experiments, and other types of voltage-dependent or ca(2+)-activated k+ channels examined were not affected [1]. | | in vivo | in an animal study, the mechanism of the genesis of tremor induced by verruculogen was investigated. it was found that atropine, glycine, mephenesin, and penicillamine with proven efficacy in relieving tremor of varied etiology, failed to modify verruculogen induced tremor. oxyaminoacetic acid, which raises the cns levels of gaba, and beta (chlorphenyl) gamma aminobutyric acid, which passes the hematoencephalic barrier, completely or partly relieved tremor and other verruculogen-induced symptoms, depending on the dose of the verruculogen administered. picrotoxin, a gaba antagonist, increased the effect of verruculogen in the tested animals [2]. | | storage | +4°C | | references | [1] knaus, h. g.,mcmanus, o.b.,lee, s.h., et al. tremorgenic indole alkaloids potently inhibit smooth muscle high-conductance calcium-activated potassium channels. biochemistry 33(19), 5819-5828 (1994).
[2] hotujac l, stern p. pharmacological examination of verruculogen induced tremor. acta med iugosl. 1974;28(3):223-9. |
| | VERRUCULOGEN Preparation Products And Raw materials |
|