- Diethylene glycol
-

- $6.00 / 1KG
-
2024-04-12
- CAS:111-46-6
- Min. Order: 1KG
- Purity: More than 99%
- Supply Ability: 500KG/Month
- DIETHYLENE GLYCOL
-

- $20.00 / 1kg
-
2024-03-13
- CAS:111-46-6
- Min. Order: 1kg
- Purity: 99.96%
- Supply Ability: 500ton
- Diethylene glycol
-
- $8.00 / 1KG
-
2024-01-22
- CAS:111-46-6
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: g-kg-tons, free sample is available
Related articles - Toxicity of Diethylene glycol
- Diethylene glycol (DEG) is a commonly used solvent and ingredient in numerous commercial products. It is used as a dehydrating....
- Jan 18,2022
|
| | Diethylene glycol Basic information |
| | Diethylene glycol Chemical Properties |
| Melting point | −10 °C(lit.) | | Boiling point | 245 °C(lit.) | | density | 1.118 g/mL at 25 °C(lit.) | | vapor density | 2.14 (vs air) | | vapor pressure | 0.01 mm Hg ( 20 °C) | | refractive index | n20/D 1.447(lit.) | | Fp | 143 °C | | storage temp. | Keep in dark place,Sealed in dry,Room Temperature | | solubility | H2O: 50 mg/mL at 20 °C, clear, colorless | | form | Oily Liquid | | pka | 14.03±0.10(Predicted) | | color | colorless | | Relative polarity | 0.713 | | PH | 5.5-7.0 (25℃, 50mg/mL in H2O) | | Odor | Practically odorless. | | explosive limit | 2-12.3% | | Water Solubility | SOLUBLE | | FreezingPoint | -10.45℃ | | Sensitive | Hygroscopic | | λmax | λ: 260 nm Amax: ≤0.02
λ: 280 nm Amax: ≤0.01 | | Merck | 14,3119 | | BRN | 969209 | | Dielectric constant | 31.7(20℃) | | Stability: | Hygroscopic | | InChIKey | MTHSVFCYNBDYFN-UHFFFAOYSA-N | | LogP | -1.98 at 20℃ | | CAS DataBase Reference | 111-46-6(CAS DataBase Reference) | | NIST Chemistry Reference | Ethanol, 2,2'-oxybis-(111-46-6) | | EPA Substance Registry System | Diethylene glycol (111-46-6) |
| Hazard Codes | Xn,T,Xi | | Risk Statements | 22 | | Safety Statements | 46 | | WGK Germany | 1 | | RTECS | ID5950000 | | F | 10 | | Autoignition Temperature | 442 °F | | Hazard Note | Toxic/Irritant | | TSCA | Yes | | HS Code | 29094100 | | Hazardous Substances Data | 111-46-6(Hazardous Substances Data) | | Toxicity | LD50 in rats, guinea pigs (g/kg): 20.76, 13.21 orally (Smyth) |
| | Diethylene glycol Usage And Synthesis |
| Chemical Properties | Diethylene glycol is a clear colorless, odorless and stable oily liquid. It is also slightly viscous, noncorrosive and nonvolatile. Because of its ether and alcohol group, diethylene glycol exhibits chemical properties characteristic of both primary alcohols and ethers. Its boiling point is considerably higher than that of ethylene glycol, and its solvent is greater. Diethylene glycol is miscible with water, ethers, lower aliphatic alcohols, aldehydes and ketones and is partially soluble in benzene, carbon tetrachloride, monobenzene, orthodichlorobenzene and toluene. It dissolves many dyes, resins, oils, nitrocellulose and many organic substances. Because of its solvent power, low volatility and hygroscopicity, it is used in textile lubricants, cutting oils, dry cleaning soap, printing inks, steam-set inks, and nongrain wood stains. In the textile industry diethylene glycol is used as a conditioning agent for wool, rayon, and cotton. As a solvent for dyes it makes a valuable assistant in dyeing and printing. The high hygroscopicity of diethylene glycol makes it an efficient softening agent for tobacco, paper, synthetic sponges, glues and casein. Diethylene glycol is especially useful in the dehydration of natural gas. A mixture of diethylene glycol and monoethanolamine will remove moisture, hydrogen sulfide and carbon dioxide from natural gas.
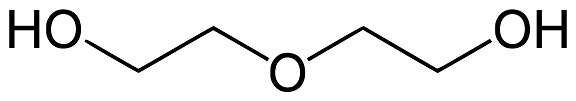
diethylene glycol structure | | Uses | Diethylene glycol (DEG) is a commonly used solvent and
ingredient in numerous commercial products. It is used as
a dehydrating agent for natural gas processing; as a lubricating
and finishing agent for textiles; a constituent in brake fluids,
lubricants, antifreeze formulations, wallpaper strippers and in
artificial fog solutions; a solvent for printing inks and textile
dyes; and is used as an intermediate in the production of some
resins, triethylene glycol, surfactants, and diethylene glycol
esters and ethers. | | Production Methods | Diethylene glycol is produced commercially as a by-product
of ethylene glycol production. It can also be produced
directly by reaction between ethylene glycol and ethylene
oxide . | | Application | Diethylene glycol has many industrial uses. It is a component of antifreeze, brake fluids, cosmetics, inks, and drying agents, and it is used as a plasticizer. In antifreeze solution for sprinkler systems, water seals for gas tanks, etc. (water with 40% diethylene glycol freezes at -18°; with 50% at -28°); as lubricating and finishing agent for wool, worsted, cotton, rayon, and silk; as solvent for vat dyes; in composition corks, glues, gelatin, casein, and pastes to prevent drying out. | | Definition | ChEBI: Diethylene glycol is a hydroxyether. | | General Description | Diethylene glycol appears as a colorless liquid. Denser than water. Contact may slightly irritate skin, eyes and mucous membranes. May be slightly toxic by ingestion. Used to make other chemicals. | | Air & Water Reactions | Slightly soluble in water. | | Reactivity Profile | Diethylene glycol is incompatible with strong oxidizing agents. Diethylene glycol is also incompatible with strong bases. Diethylene glycol can react with sulfuric acid and other dehydrating agents, nitric acid, oxygen, hydrogen peroxide, perchloric acid and strong acids. Mixtures with sodium hydroxide decompose exothermically when heated to 446° F. | | Health Hazard | Ingestion of large amounts may cause degeneration of kidney and liver and cause death. Liquid may cause slight skin irritation. | | Fire Hazard | Diethylene glycol is combustible. | | Flammability and Explosibility | Non flammable | | Safety Profile | Moderately toxic to
humans by ingestion. Poison experimentally
by inhalation. Moderately toxic by ingestion
and intravenous routes. Questionable
carcinogen with experimental carcinogenic,tumorigenic, and teratogenic data. An eye
and human skin irritant. Combustible when
exposed to heat or flame; can react with
oxidning materials. To fight fire, use alcohol
foam, water, Con, dry chemical. Mixtures
with sodium hydroxide decompose
exothermically when heated to 230℃ and
release explosive hydrogen gas. When
heated to decomposition it emits acrid
smoke and irritating fumes. See also
GLYCOL ETHERS. | | Toxicology | The toxicity of diethylene glycol is similar to ethylene glycol and clearly is a CNS depressant. It has a low inhalation hazard because of its low vapor pressure; however, inhalation of the mist or aerosol is to be avoided. Workplace levels for vapors and aerosols cannot exceed 50 ppm. In case of accidental release of diethylene glycol, use of a full-face positive air pressure respirator is recommended. Even though the toxicokinetics in humans is not completely understood, its toxic nature is confirmed by animal studies. Several human cases were reported in the medical literature. Several children in Haiti died in 1995 and 1996 following the consumption of medication containing diethylene glycol. Similar other cases in children were reported in other countries as well. A 24-year-old man developed encephalopathy and rapidly became quadriplegic following ingestion of a solution containing diethylene glycol . Thus, the toxicity of diethylene glycol is well established. | | Carcinogenicity | Weil et al. , in their longterm
studies on rats of three different age levels, found only
one bladder tumor in those fed diets that contained 4%
diethylene glycol. This tumor was in a rat that also had
bladder stones . To clarify the question of the cause of
the tumor, Weil et al. implanted calcium oxalate
stones or glass beads into the bladders of rats. They found that
bladder tumors never developed without the presence of a
foreign body in the bladder. This led to the conclusion that
diethylene glycol essentially free of ethylene glycol is not a
primary carcinogen. | | Environmental Fate | Diethylene glycol is metabolized by alcohol dehydrogenase to
toxic metabolites predominantly, HEAA and DGA. DEG can
cause an anion gap metabolic acidosis, cortical necrosis
resulting in permanent renal failure and neurotoxicity. DGA,
not HEAA, was recently identified as being the primary nephrotoxic agent causing proximal tubule cell death. The
neurotoxicity seen after DEG poisoning is only recently
described. The neurotoxicity is delayed and has cranial and
peripheral demyelinating sensorimotor polyneuropathy
pattern. The exact mechanism of the neurotoxicity remains
unclear and in the cases described in the literature, it appears to
be prolonged but does show evidence of reversibility. | | Toxicity evaluation | Diethylene glycol is miscible with water, has a low vapor
pressure of 0.008 hPa at 25°C, a very low log Kow of �1.98, and also a low Koc. Consequently, water is the most relevant environmental
compartment. Calculation according to Mackay,
Level I indicates the following distribution among environmental
compartments: air 0.75%, water 99.25%, soil 0%,
sediment 0%; confirming the relevance of the pelagic systems.
The substance is readily biodegradable and the very low
log Kow suggests a low potential for bioaccumulation. |
| | Diethylene glycol Preparation Products And Raw materials |
| Raw materials | ETHYLENE OXIDE | | Preparation Products | Toluene-->Benzene-->Xylene-->Ethylene glycol-->Morpholine-->Casein-->4-Benzylpiperidine-->4-Methylmorpholine-->2-Chloro-4-dodecylphenol-->Unsaturated polyester resin-->4-(4-Methoxyphenyl)butyric acid-->6-Phenylhexanoic acid-->2,6-Dichloroindophenol sodium salt-->4-(2-THIENYL)BUTYRIC ACID-->1-PYRENEBUTYRIC ACID-->1-PYRENEDECANOIC ACID-->2,3-DIHYDROXYQUINOXALINE-6-CARBOXYLIC ACID-->Diethylene Glycol Monoethyl Ether-->Ursodeoxycholic acid-->4-Amino-2,6-dichlorophenol-->polyurethae finishes PUC series-->defoaming agent OTD-->BT modified acrylic resin binder series-->CSF series modified sacrylic binder-->AC anti-fungus leather finishing agent-->2-Ethoxyethyl ether-->weather-proof acrylic binder series-->21-Iodo-16-methylpregna-1,4,9(11)-trien-17-ol-3,20-dione-->N-Ethylmorpholine-->1,3-DIFLUORO-2-PROPANOL-->thickening agent PAS-->16-Methylpregna-1,4,9(11)-trien-17-ol-3,20-dione-->1-CHLORO-3-FLUOROISOPROPANOL-->2-(2-HEXYLOXYETHOXY)ETHANOL-->Disperse Yellow 126-->2,2'-OXYDIACETYL CHLORIDE-->polyurethane water-based emulsion finishes PU-II series-->diamfenetide-->Diethylene Glycol Dibutyl Ether-->Diethylene glycol dibenzoate |
|