- (-)-Phenylephrine Base
-

- $50.00 / 10kg
-
2023-07-31
- CAS:59-42-7
- Min. Order: 20kg
- Purity: 98%
- Supply Ability: 5000ton
- Phenylephrine
-

- $0.00 / 1Kg/Bag
-
2021-09-29
- CAS:59-42-7
- Min. Order: 1KG
- Purity: 99.0%~100.5%; EP9.0
- Supply Ability: 1000kg/month
- Phenylephrine
-

- $15.00 / 1KG
-
2021-08-12
- CAS:59-42-7
- Min. Order: 1KG
- Purity: 99%+ HPLC
- Supply Ability: Monthly supply of 1 ton
|
| | Phenylephrine Basic information |
| Product Name: | Phenylephrine | | Synonyms: | component of Dristan cold;component of Dristan nasal mist;component of Entex;component of Histalet forte;component of Hycomine compound;component of Prefrin-a;component of Pv tussin syrup;component of Relief | | CAS: | 59-42-7 | | MF: | C9H13NO2 | | MW: | 167.21 | | EINECS: | 200-424-8 | | Product Categories: | | | Mol File: | 59-42-7.mol | 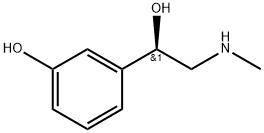 |
| | Phenylephrine Chemical Properties |
| Melting point | 171°C | | Boiling point | 295.79°C (rough estimate) | | density | 1.1222 (rough estimate) | | refractive index | -55.5 ° (C=5, 1mol/L HCl) | | storage temp. | -20°C Freezer, Under inert atmosphere | | solubility | Slightly soluble in water, sparingly soluble in methanol, slightly soluble in ethanol (96 per cent). It dissolves in dilute mineral acids and in dilute solutions of alkali hydroxides. | | pka | pKa 8.9 (Uncertain) | | color | White to Off-White | | CAS DataBase Reference | 59-42-7(CAS DataBase Reference) | | NIST Chemistry Reference | Benzenemethanol, 3-hydroxy-«alpha»-[(methylamino)methyl]-, (r)-(59-42-7) |
| | Phenylephrine Usage And Synthesis |
| Description | This synthetic drug has both chemical and pharmacological similarities to norepinephrine.
A characteristic quality of phenylephrine is the distinctly expressed selectivity to α-
adrenoreceptors, especially α1-adrenoreceptors. Although phenylephrine increases the
contractibility of blood vessels, in practical terms it is not considered a cardiostimulant. | | Chemical Properties | White or almost white, crystalline powder. | | Uses | L-Phenylephrine is an adrenergic α1A receptor agonist (Ki = 1.4 μM) that demonstrates selectivity against the α1B and α1C receptor subtypes (Kis = 23.9 and 47.8 μM, respectively). By stimulating adrenergic α1 receptors, L-phenylephrine can induce aortic smooth muscle contractions, although reported relative affinity and potency values in rabbit are 5-fold weaker compared to that of L-norepinephrine. This compound is frequently used to precontract smooth muscle in preparations designed to study the properties of various vasodilator agents. Because L-phenylephrine acts on adrenergic α1 receptors in the arterioles of the nasal mucosa to produce constriction, it has been examined clinically as an oral decongestant. | | Uses | Phenylephrine is used in hypotension, paroxysmal supraventricular tachycardia, and
shock. It is also used locally, particularly in the form of nasal spray, for relieving edema. | | Definition | ChEBI: A member of the class of the class of phenylethanolamines that is (1R)-2-(methylamino)-1-phenylethan-1-ol carrying an additional hydroxy substituent at position 3 on the phenyl ring. | | Brand name | Afrin 4 Hour Nasal Spray (Schering-Plough
Health Care); Biomydrin (Parke-Davis); Mydfrin (Alcon);
Neo-Synephrine (Sterling Health U.S.A.); Nostril (Boehringer
Ingelheim);Fenox;Forte;Isopto;Minims;Visadron. | | General Description | (Neo-Synephrine, a prototypical selectivedirect-acting 1-agonist) differs from E only inlacking a p-OH group. It is orally active, and its DOA isabout twice that of E because it lacks the catechol moietyand thus is not metabolized by COMT. However, its oralbioavailability is less than 10% because of its hydrophilicproperties (log P=0.3), intestinal 3 -O-glucuronidation/sulfation and metabolism by MAO. Lacking the p-OHgroup, it is less potent than E and NE but it is a selectiveα1-agonist and thus a potent vasoconstrictor. It is usedsimilarly to metaraminol and methoxamine for hypotension.Another use is in the treatment of severe hypotensionresulting from either shock or drug administration. It alsohas widespread use as a nonprescription nasal decongestantin both oral and topical preparations. When applied tomucous membranes, it reduces congestion and swelling byconstricting the blood vessels of the membranes. In theeye, it is used to dilate the pupil and to treat open-angleglaucoma. In addition, it is used in spinal anesthesia toprolong the anesthesia and to prevent a drop in blood pressureduring the procedure. It is relatively nontoxic and produceslittle CNS stimulation. Metaraminol is just anotherexample. | | Clinical Use | Phenylephrine is a potent direct-acting α1-agonist with clinical effects similar
to those of noradrenaline. It causes widespread vasoconstriction with an
increase in arterial pressure, reflex bradycardia and decrease in cardiac
output. It may be administered by i.v. bolus (50–100μg boluses) and i.v.
infusion (50–150μgmin –1) to maintain arterial pressure during general
anaesthesia or other causes of low SVR. It may also be used topically as a
nasal decongestant or mydriatic. There is some evidence suggesting a
paradoxical reduction in cerebral oxygen delivery. | | Safety Profile | Poison by ingestion,
subcutaneous, intravenous, intraperitoneal,
and intraduodenal routes. Human systemic
effects by ocular route: blood pressure
increase. An experimental teratogen. Other
experimental reproductive effects. A nasal
decongestant. When heated to
decomposition it emits toxic fumes of NOx. | | Synthesis | Phenylephrine, 1-(3-hydroxyphenyl)-2-methylaminoethanol (11.1.16),
which differs from epinephrine, in that it does not have a hydroxyl group at C4 of the aro�matic ring, is synthesized by an analogous scheme of making epinephrine; however,
instead of using ω-chloro-3,4-dihydroxyacetophenone, ω-chloro-3-dihydroxyacetophe�none is used [11,22,23]. | | Veterinary Drugs and Treatments | Phenylephrine has been used to treat hypotension and shock (after
adequate volume replacement), but many clinicians prefer to use
an agent that also has cardiostimulatory properties. Phenylephrine
is recommended for use to treat hypotension secondary to drug
overdoses or idiosyncratic hypotensive reactions to drugs such as
phenothiazines, adrenergic blocking agents, and ganglionic blockers.
Its use to treat hypotension resulting from barbiturate or other
CNS depressant agents is controversial. Phenylephrine has been
used to increase blood pressure to terminate attacks of paroxysmal
supraventricular tachycardia, particularly when the patient is also
hypotensive. Phenylephrine has been used to both treat hypotension
and prolong the effects of spinal anesthesia.
Ophthalmic uses of phenylephrine include use for some diagnostic
eye examinations, reducing posterior synechiae formation,
and relieving pain associated with complicated uveitis. It has been
applied intranasally in an attempt to reduce nasal congestion. | | in vitro | in neonatal rat cardiomyocytes, 50 μm l-phenylephrine treatment could protect cells from apoptosis induced by hypoxia (95% n2 and 5% co2) and serum deprivation through α-adrenergic receptor stimulation [2]. besides, in neural progenitor cells (npcs), 10 μm l-phenylephrine could increase npcs proliferation by approximately 160% [3]. furthermore, in cultured rat neonatal cms (ncms), l-phenylephrine could increase cross-sectional area, and significantly increase il-6 mrna levels, while decreasing pgc1α mrna levels [4]. | | in vivo | studies in sprague-dawley male rats found that, local infiltration of l-phenylephrine could induce cutaneous anesthesia in a dose dependent manner, which could be significantly inhibited by α1-adrenergic receptor antagonists [5]. | | target | adrenergic α1a receptor | | references | [1] lomasney j w, cotecchia s, lorenz w, et al. molecular cloning and expression of the cdna for the alpha 1a-adrenergic receptor. the gene for which is located on human chromosome 5.[j]. journal of biological chemistry, 1991, 266(10): 6365-6369.
[2] zhu h, mcelweewitmer s, perrone m, et al. phenylephrine protects neonatal rat cardiomyocytes from hypoxia and serum deprivation-induced apoptosis.[j]. cell death & differentiation, 2000, 7(9): 773-784.
[3] hiramoto t, ihara y, watanabe y, et al. α-1 adrenergic receptors stimulation induces the proliferation of neural progenitor cells in vitro[j]. neuroscience letters, 2006, 408(1): 25-28.
[4] planavila a, redondo i, hondares e, et al. fibroblast growth factor 21 protects against cardiac hypertrophy in mice[j]. nature communications, 2013.
[5] shieh j, chu c, wang j, et al. epinephrine, phenylephrine, and methoxamine induce infiltrative anesthesia via α1-adrenoceptors in rats[j]. acta pharmacologica sinica, 2009, 30(9): 1227-1236.
[6] hatton r c, winterstein a g, mckelvey r p, et al. efficacy and safety of oral phenylephrine: systematic review and meta-analysis[j]. annals of pharmacotherapy, 2007, 41(3): 381-390. |
| | Phenylephrine Preparation Products And Raw materials |
|