- Isoproturon
-

- $80.00 / 1kg
-
2024-04-22
- CAS:34123-59-6
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 1000
- Isoproturon
-

- $0.00 / 25KG
-
2023-11-24
- CAS:34123-59-6
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 50000KG/month
- Isoproturon
-

- $0.00 / 1KG
-
2023-09-06
- CAS:34123-59-6
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 500000kg
|
| | Isoproturon Chemical Properties |
| | Isoproturon Usage And Synthesis |
| Identification | English general name: Isoproturon(BSI,ISO)
Other names: HOE16410; CGA-18731; DPX6774; LS69-1299;IP50;AE F016410;35689RP
Chemical name: 3- (4-isopropylphenyl)-1,1-dimethylurea
| | Structural formula | 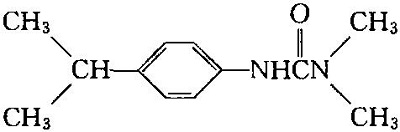
The physicochemical properties of this product are colorless crystallization, and the melting point is 158 degrees C. The vapor pressure is 3.15 x 10-3mPa (20 C) and 8.1 x 10-3mPa (25 C). The solubility in different solvents (20 C):22 C, 65mg/L in water, 75g/L in methanol, 63g/L in dichloromethane, 38g/L in acetone, 5g/L in benzene, 0.2g/L in hexane, 4g/L in xylene. Industrial product purity is more than 98.5% and the melting point is 153~156℃. Stable to light, acid and alkali. In strong acid and strong alkali, it can be hydrolyzed to two methylamine and corresponding aromatic amines. | | Toxicity | Acute oral administmiceion of LD50 is1826 to 2417mg/kg in mice and 3350mg/kg in mice. No irritation to the eyes and skin. Acute inhalation of LC50 (4 hours) is more than 1.95mg/L air in mice. No action dose for 90 days: mice 400mg/kg feed and dog 50mg/kg feed. No action dose is 80mg/kg feed for two year feeding test in mice. ADI to people is 0.0062mg/kg weight. Non mammal acute oral administration of LD50: Japanese quail 3042 ~ 7926mg/kg, dove >5g/kg. Ichthyism LC50 (96 hours): Golden orfe 129mg/L, blue gill >100mg/L, rainbow trout 90mg/L, rainbow trout 37mg/L, carp 193mg/L, catfish 9mg/L. It's poisonous to bees. Water flea LC50 (48 hours) 507mg/L, seaweed LC50 (72 hours) 0.03mg/L.
| | Applicable crops | It is suitable for crops such as crop wheat, barley, cotton, peanut, corn, rice, potato, soybean, pea, Vicia bean etc.. | | Mechanism of action | Isoproteron is a substitute for urea selective pre and post buds herbicides.It is mainly absorbed by the root of the clutter, and are less absorbed by the stems and leaves. It will move upward with the water in in the catheter. Many of them are distributed on the tip of the leaf and the leaf edge, and play a role in green cells. It is an inhibitor of the electron transfer of photosynthesis, which interferes with the normal conduct of photosynthesis. In light of light, oxygen and carbon dioxide can not be released, organic matter is stopped, and sensitive weeds are starved to death.
| | Prevention and control object | Crabgrass, Chenopodium, Alopecurus, bluegrass, ryegrass, and blue violet, Tian lady's-thumb mustard, Tian Ju, knotgrass, spurrey, chickweed and Atriplex, millet grass genus, Amaranthus, wind Agrostis, Centaurea etc..
| | How to use it | Mainly through the root absorption, can be used as the soil treatment after sowing and before seedling, and can also be used to treat stem and leaf after seedling. Take the wheat field as an example; the pre-treating usually takes place after sowing and before seeding of wheat or barley. With 75% isopropane wet powder 1.5 ~ 2.0kg per hectare, the water will be added at 600 kg evenly sprayed on the soil surface. The post - seedling treatment is usually in the period of 3 leaves of wheat or barley to the early tillering stage. Field weeds are in the 2~5 leaf stage. 75% wet powder per hectare was 1.3 ~ 2kg, and the water is about 600kg, evenly sprayed on the weed stem and leaf. The drug has a half-life in the soil for 20 days, effective for 2~3 months in autumn.
| | Precautions for use |
- The land applied with calcium superphosphate cannot be used.
- It is not suitable to apply the soil with weak or frozen crop growth, leaking ploughing and bad sand or poor drainage.
| | Synthesis in Phosgene Method | 1. For isopropyl benzene with nitric acid, nitrite and isopropyl benzene, and again or against amino isopropyl benzene.
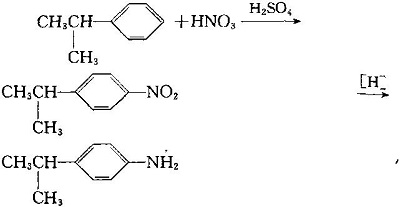
2. Synthesis of isopropane isocyanate by reaction of amino isopropyl benzene with phosgene
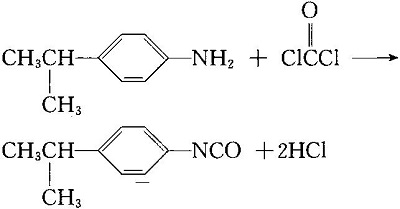
3. Isopropane isocyanate reacts with dimethyl ester to produce isoproteron.
 | | Synthesis in Non-phosgene method | Reaction with isopropyl aniline with urea instead of phosgene in aqueous solution, forming an intermediate isopropylureas.Then the reaction continues in the dimethylamine agueous solution to get isoproteron with a total yield of 76%.
Under the catalysis of inorganic alkali, the isopropyl three chloroacetanilide and dimethylamine react at 60~80 centigrade for 30 minutes, and the high yield (95%) of isopalon will be obtained
.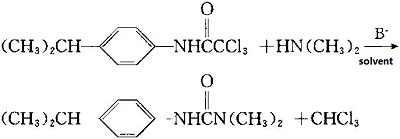
a. Preparation of isopropyl three chloroacetanilide:
13.5g (0.10mol) trichloroacetic acid is added to the reaction flask, stirring and dropping 14.0g (0.10mol) trichloride.The tempemiceure is controlled at 100~110 degrees centigrade. After dropping, the constant tempemiceure reaction lasts for 30 minutes. And then add the 100ml ice water, vacuum filtered, collect solid and wash it with water to neutral. The white crystal is obtained by recrystallization with ethanol / water. The yield is 91% and the melting point is 123~125℃.
b. Synthesis of isoproteron:
14.0g (0.05mol) p-isopropyl three chloroacetanilide, 5.0g (0.125mol) sodium hydroxide (anhydrous Na2CO3 or K2CO3, yield will be 91% and 92.5%), 4.5g (0.10mol)dimethylamine ( ethanol concentmiceion)and 80ml dimethyl sulfoxide(also two methyl amine or methyl cyanide) are put into the reactor before stirring and heating for 30 minutes. The tempemiceure is controlled at 60~80 degrees centigrade (with the increase of the reaction tempemiceure, the color of the product deepens and the quality decreases).After the end of the reaction, the reaction liquid is poured into the water.After vacuum filter and collection of the solid, recrystallization occurs with ethanol / water to obtain white solids. The yield is 95%, and the melting point is 152~154degrees centigrade.
| isopropyl benzene | 1.04 | Phosgene (80%) | 0.84 | | dimethylbenzene | 0.93 | Sulphuric acid (93%) | 2.24 | | dimethylamine | 0.95 | Nitric acid (9%) | 0.67 |
| | Analysis method | High performance liquid chromatography (HPLC), ultraviolet detector UV- 254nm, Chromatographic column: S:100, 5 mu, 250 x 4.6 (I.D) mm, mobile phase: n-heptane chloroform (1% acetic acid) - ethanol =70:15:1. The flow micee is 3.0ml/ minutes.Retention time: 7.5 minutes for isoprotrol. Internal standard (acetyl aniline) for 12 minutes. The prepamiceion of the solution: The standard products and samples containing ISO proteron 50mg are added to the 24mg internal standard, and the two chloro methyl was dissolved in the 50ml volume bottle. Or use the mobile phase: cyclohexane - isopropanol =90:10, flow micee of 1.5ml/ minutes, Retention time: internal standard (acetanilide, 2.5mg/ml mobile phase) 6.55 minutes, isoproteron for 9.52 minutes. Preparation of solution: take the standard products and samples containing iso - proteron of 500mg, respectively.First, 10ml isopropanol is uded to get it dissolved and cyclohexane is used to get it dissolved in 100ml. The solution of 5.0ml is taken out together with the internal standard solution of 5.0ml. Get it dissolved in a 50ml volumetric bottle with a mobile phase.
| | Chemical Properties | White Solid | | Uses | Pre-and post-emergence herbicide for control of annual grasses and broad-leaved weeds. Herbicide. | | Uses | Isoproturon may be used as a reference standard for the determination of isoproturon in crops using high performance liquid chromatography (HPLC) with fluorescence detection combined with UV decomposition and post-column derivatization. | | Definition | ChEBI: A member of the class of ureas that is 1,1-dimethylurea substituted by a p-cumenyl group at position 3. A selective, systemic herbicide used to control annual grasses and broadleaf weeds in cereals, its use within the EU has been banned after
eptember 2017 on the grounds of potential groundwater contamination and risks to aquatic life; there have also been concerns about its endocrine-disrupting properties. | | General Description | Isoproturon is a phenylurea herbicide used to control ryegrass, silky bentgrass, and broadleaf species in spring and winter wheat. | | Hazard | Moderately toxic by ingestion, inhalation,
and skin contact. | | Metabolic pathway | The major degradation product of isoproturon in most
soil samples is identified as 2-hydroxyisoproturon, and
the polar metabolites characterized are
monodesmethylisoproturon and 2-
hydroxymonodesmethylisoproturon. The detection of
isoproturon in soil solution down to 170 cm depth and
in creek water in concentrations exceeding 4 μg l-1
and also of the polar metabolites in concentrations up
to 0.9 μg l-1 indicates the mobility of this phenylurea
herbicide and its degradation products. |
| | Isoproturon Preparation Products And Raw materials |
|