|
ChemicalBook Optimization Suppliers |
|
| | ラミブジン Usage And Synthesis |
| 外観 | 白色〜わずかにうすい褐色, 結晶〜粉末 | | 溶解性 | ジメチルスルホキシドに溶けやすく、水にやや溶けやすく、メタノール及びエタノールにやや溶けにくく、ジエチルエーテルにほとんど溶けない。 | | 用途 | 核酸アナログ逆転写酵素阻害
剤です。ウィルス DNA ポリメラーゼによる
基質の取り込みを競合的に阻害し、DNA 鎖の
伸長を停止することにより、ウィルスの増殖
阻害作用を示します。
| | 用途 | 核酸アナログ逆転写酵素阻害
剤です。ウイルス DNA ポリメラーゼによる
基質の取り込みを競合的に阻害し、DNA 鎖の
伸長を停止することにより、ウイルス増殖阻
害作用を示します。 | | 効能 | 抗ウイルス薬, 逆転写酵素阻害薬 | | 商品名 | エピビル (ヴィーブヘルスケア); ゼフィックス (グラクソ・スミスクライン) | | 説明 | Lamivudine is a new generation orally active nucleoside analog launched in the U.S.A. for use in combination with zidovudine (AZT) as a first-line therapy for patients with HIV infection. Lamivudine is rapidly converted to phosphorylated metabolites in the body which act as inhibitors and chain terminators of HIV reverse transcriptase (RT), the enzyme required for the replication of the HIV genome. Lamivudine has similar inhibitory potency to RT as AZT but is 10 times less toxic and is active against AZT-resistant strains of HIV. | | 化学的特性 | White solid from methanol-ethyl acetate. [α]D21-132° (C=1.08, methanol). Or crystallised from boiling ethanol, melting point 160-162°C. [α]D21-135° (C=0.38, methanol). | | Originator | BioChem Pharma (Canada) | | 来歴 | Lamivudine[134678-17-4] is produced by GlaxoSmithKline LLC. In the early 1990s, it was used by some countries in Europe and North America to treat AIDS. In the mid-1990s, medical experts found that it had an inhibitory effect on the DNA of hepatitis B virus. In 1998, the US Food and Drug Administration (FDA) first approved it as a treatment drug for hepatitis B. In 1999, the China Food and Drug Administration approved this drug as a hepatitis B treatment drug, with the Chinese trade name "Heputin". | | 使用 | Lamivudine (Epivir-HBV) is in a class of medications called nucleoside reverse transcriptase inhibitors (NRTIs). It works by decreasing the amount of HIV and hepatitis B in the blood. It is used to treat hepatitis B infection. It is also used along with other medications to treat human immunodeficiency virus (HIV) infection in adults and children 3 months of age and older. | | 定義 | ChEBI: Lamivudine is a monothioacetal that consists of cytosine having a (2R,5S)-2-(hydroxymethyl)-1,3-oxathiolan-5-yl moiety attached at position 1. An inhibitor of HIV-1 reverse transcriptase. | | 合成 | Aqueous hydrochloric acid (6N, 30 ml) was slowly added to a solution of 20 gm of the solid (2R-cis)-4-Amino-1-[2-(hydroxymethyl)-1,3-oxathiolan-5-yl]-2(1H)-pyrimidi- none.S-1,1'-binaphthyl-2,2'-diyl hydrogen phosphate in water (200 ml) at 45-50 deg C. Stirred the reaction for 1 hour at room temperature. The solid S-1,1'-binaphthyl-2,2'-diyl hydrogen phosphate was filtered and the aqueous layer was neutralized with aqueous sodium hydroxide solution (30%, 20 ml). The solvent was recovered under vacuum at 40-45 deg C., the product obtained was dissolved in methanol (200 ml), filtered to remove the inorganic salts, the filtrate was concentrated under vacuum at 40-45 deg C. and the residual solid obtained was dissolved in ethanol (50 ml), heated to 50 deg C., slowly allowed to room temperature, cooled to 10 deg C., filtered and dried at 40-45 deg C. to obtain 5 gm of Lamivudine(Chiral purity: 97.5%).
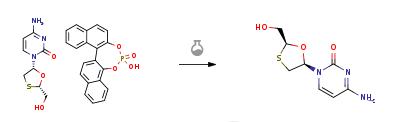 | | brand name | Epivir (GlaxoSmithKline). | | 獲得抵抗性 | Long-term lamivudine administration frequently elicits viral resistance characterized by a reincrease of viral replication in an adherent patient. The incidence of lamivudine resistance is 14% to 32% after 1 year of treatment, 38% after 2 years, and 53% to 76% after 3 years. | | 薬物動態学 | The pharmacokinetics of lamivudine are similar in patients with HIV-1 or HBV infection, and healthy volunteers. The drug is rapidly absorbed after oral administration, with maximum serum concentrations usually attained 0.5 to 1.5 hours after the dose. | | 臨床応用 | Lamivudine is indicated for the treatment of HIV when
used in combination with other antiretroviral agents.A
lower dose than that used to treat HIV is approved for
the treatment of HBV. | | 副作用 | Lamivudine oral tablet can cause mild or serious side effects. The more common side effects that can occur with lamivudine include:
cough,diarrhea,fatigue,headache,malaise (general discomfort),nasal symptoms, such as a runny nose,nausea. | | 薬物相互作用 | There are potentially dangerous interactions with other drugs when used in combination.
Antimicrobials: trimethoprim inhibits the excretion of lamivudine.
Antivirals: avoid concomitant use with foscarnet, emtricitabine, and IV ganciclovir
Cytotoxic drugs: avoid concomitant use with clarithromycin.
Orlistat: absorption may be reduced by orlistat. | | 代謝 | Lamivudine is metabolised intracellularly to the active antiviral triphosphate. Hepatic metabolism is low (5-10%) and the majority of lamivudine is excreted unchanged in the urine via glomerular filtration and active secretion (organic cationic transport system). | | 参考文献 | [1] ROUSSOSA. Lamivudine treatment for acute severe hepatitis B: report of a case and review of the literature.[J]. Acta gastro-enterologica Belgica, 2008, 71 1: 30-32.
[2] ARTSE J WainbergM A. Mechanisms of nucleoside analog antiviral activity and resistance during human immunodeficiency virus reverse transcription.[J]. Antimicrobial Agents and Chemotherapy, 1996, 40 3: 527-540. DOI:10.1128/AAC.40.3.527.
[3] COATESJ A. (-)-2’-deoxy-3’-thiacytidine is a potent, highly selective inhibitor of human immunodeficiency virus type 1 and type 2 replication in vitro.[J]. Antimicrobial Agents and Chemotherapy, 1992, 36 4: 733-739. DOI:10.1128/AAC.36.4.733.
[4] KONGHUI. Targeted P2X7/NLRP3 signaling pathway against inflammation, apoptosis, and pyroptosis of retinal endothelial cells in diabetic retinopathy.[J]. Cell Death & Disease, 2022, 13 4: 336. DOI:10.1038/s41419-022-04786-w.
[5] KUMAR P N, PATEL P. Lamivudine for the treatment of HIV[J]. Expert Opinion on Drug Metabolism & Toxicology, 2010, 6: 105-114. DOI:10.1517/17425250903490418.
[6] PERRYC M FauldsD. Lamivudine. A review of its antiviral activity, pharmacokinetic properties and therapeutic efficacy in the management of HIV infection.[J]. Drugs, 1997, 53 4: 657-680. DOI:10.2165/00003495-199753040-00008.
[7] RAJURKARMIHIR. Reverse Transcriptase Inhibition Disrupts Repeat Element Life Cycle in Colorectal Cancer.[J]. Cancer discovery, 2022, 12 6: 1462-1481. DOI:10.1158/2159-8290.CD-21-1117. |
|