- TOMATINE
-

- $0.00 / 25KG
-
2023-08-11
- CAS:17406-45-0
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 10000kg/monnth
- TOMATINE
-

- $10.00 / 1KG
-
2023-04-05
- CAS:17406-45-0
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 10 mt
- Tomatine
-
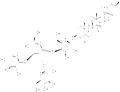
- $0.00 / 5mg
-
2023-02-24
- CAS:17406-45-0
- Min. Order: 5mg
- Purity: ≥98%(HPLC)
- Supply Ability: 10 g
|
| | TOMATINE Chemical Properties |
| Melting point | 300-305°C | | alpha | D20 -18° (c = 0.55 in pyridine) | | Boiling point | 806.69°C (rough estimate) | | density | 1.48±0.1 g/cm3 (20 ºC 760 Torr) | | refractive index | 1.5280 (estimate) | | storage temp. | 2-8°C | | solubility | DMSO (Slightly), Methanol (Slightly, Heated), Pyridine (Slightly) | | pka | 12.77±0.70(Predicted) | | form | Solid | | color | White to Off-White | | Merck | 14,9545 | | LogP | 2.220 (est) | | CAS DataBase Reference | 17406-45-0 |
| | TOMATINE Usage And Synthesis |
| Health Benifits | Tomatine (sometimes called tomatin or lycopersicin) is a glycoalkaloid, found in the stems and leaves of tomato plants, and in the fruits at much lower concentrations. It has fungicidal, antimicrobial, and insecticidal properties. Chemically pure tomatine is a white crystalline solid at standard temperature and pressure. Tomatine, as well as the closely related aglycon (or aglycone) derivative tomatidine have been shown to have multiple health benefits.
Tomatine is a steroid alkaloid that is tomatidine in which the hydroxy group at position 3 is linked to lycotetraose, a tetrasaccharide composed of two units of D-glucose, one unit of D-xylose, and one unit of D-galactose. It has a role as an immunological adjuvant, a phytotoxin and an antifungal agent. It is a steroid alkaloid, a tetrasaccharide derivative, an alkaloid antibiotic and a glycoside. It derives from a tomatidine.
| | Tomatine Toxin Symptoms | The possible risks of tomatine for humans have not been formally studied, so no NOAEL can be deduced. The toxicity of tomatine has only been studied on laboratory animals. The symptoms of acute tomatine poisoning in animals are similar to the symptoms of poisoning by solanine, a potato glycoalkaloid. These symptoms include vomiting, diarrhea, abdominal pain, drowsiness, confusion, weakness, and depression. Generally, tomatine is regarded to cause less toxic effect to mammals than other alkaloids such as solanine. The amount of tomatine absorbed by the human body as well as the possible metabolism is unknown. There is no evidence that consumption of tomatoes causes acute toxic or genotoxic effects.
| | Description | This glycosidic alkaloid has been obtained from the dried leaves of Lycopersicum
pimpinelli/olium (Syn. Solanum racemigerum) and also occurs in L. esculentum;
L. hirsutum; L. peruvianum; L. peruvianum var. chutatum and L. putatum. The
alkaloid forms colourless needles from MeOH and has a somewhat indefinite
melting point about 270°C. It is laevorotatory with [α]20D - 30° (pyridine) and
on hydrolysis with H2S04 yields tomatidine, I mole of galactose, 2 moles of
glucose and 1 mole of xylose. The tetrasaccharide unit is known as lycotetraose.
Partial hydrolysis with acid yields a series of products depending upon which,
and how many, of the sugar residues are split off from the molecule. Those
which have been isolated are: tomatidine lycotriose I (galactose-glucose-glucose);
tomatidine lycotriose II (galactose-glucose-xylose); tomatidine lycobiose
(galactose-glucose) and tomatidine-galactose. | | Chemical Properties | Faintly beige powder | | Uses | Tomatine is used as an antiproliferatice agent used in the suppression of breast adenocarcinoma cells. | | Uses | Precipitating agent for steroids. | | Definition | ChEBI: A steroid alkaloid that is tomatidine in which the hydroxy group at position 3 is linked to lycotetraose, a tetrasaccharide composed of two units of D-glucose, one unit of D-xylose, and one unit of D-galac
ose. | | target | NF-kB | PI3K | Akt | ERK | MMP(e.g.TIMP) | AP-1 | ROS | Calcium Channel | | Purification Methods | Tomatine is recrystallised from MeOH, EtOH, aqueous EtOH or dioxane/NH3. It is almost insoluble in pet ether, Et2O or H2O. [Reichstein Angew Chem 74 887 1962, Beilstein 27 III/IV 1954.] | | References | Fontaine et al., Arch. Biochem., 18,467 (1948)
Kuhn, Low., Ber., 81, 552 (1948)
Kuhn, Low, Gauhe., ibid, 83,448 (1950)
Fontaine et al., Arch. Biochem., 27,461 (1950)
Fontaine et al., J. Amer. Chern. Soc., 73, 878 (1951)
Kuhn, Low, Trischmann., Angew. Chern., 68, 212 (1956)
Kuhn et al., Ber., 90, 203 (1957) |
| | TOMATINE Preparation Products And Raw materials |
|