- Telithromycin
-

- $10.50 / 1KG
-
2023-03-06
- CAS:191114-48-4
- Min. Order: 1KG
- Purity: 99
- Supply Ability: 1 ton
- Telithromycin
-

- $0.00 / 1G
-
2023-02-09
- CAS:191114-48-4
- Min. Order: 1G
- Purity: 98%min
- Supply Ability: 30kg/month
- Telithromycin
-

- $10.00 / 1Kg/Bag
-
2021-11-04
- CAS:191114-48-4
- Min. Order: 1Kg/Bag
- Purity: 99%
- Supply Ability: 20 Tons
|
| Product Name: | Telithromycin | | Synonyms: | (1R,2R,4R,6S,7R,8R,10R,13R,14S)-7-[(2S,3R,4S,6R)-4-Dimethylamino-3-hydroxy-6-methyloxan-2-yl]oxy-13-ethyl-6-methoxy-2,4,6,8,10,14-hexamethyl-17-[4-(4-pyridin-3-ylimidazol-1-yl)butyl]-12,15-dioxa-17-azabicyclo[12.3.0]heptadecane-3,9,11,16-tetrone;KETEK;(3aS,4R,7R,9R,10R,11R,13R,15R,15aR)-4-Ethyloctahydro-11-Methoxy-3a,7,9,11,13,15-hexaMethyl-1-[4-[4-(3-pyridinyl)-1H-iMidazol-1-yl]butyl]-10-[[3,4,6-trideoxy-3-(diMethylaMino)-β-D-xylo-hexopyranosyl]oxy]-2H-oxacyclotetradecino[4,3-d]oxazole-2,6,8,14(1H,7H,9H)tetrone;HMR 3647;RU 66647;2H-Oxacyclotetradecino[4,3-d]oxazole-2,6,8,14(1H,7H,9H)-tetrone,4-ethyloctahydro-11-Methoxy-3a,7,9,11,13,15-hexaMethyl-1-[4-[4-(3-pyridinyl)-1H-iMidazol-1-yl]butyl]-10-[[3,4,6-trideoxy-3-(diMethylaMino)-b-D-xylo-hexopyranosyl]oxy]-,(3aS,4R,7R,9R,10R,11R,1;TELITHROMYCIN;TelithroMycin 90% | | CAS: | 191114-48-4 | | MF: | C43H65N5O10 | | MW: | 812 | | EINECS: | 682-750-4 | | Product Categories: | Amines;Aromatics;Carbohydrates & Derivatives;Heterocycles;Intermediates & Fine Chemicals;Pharmaceuticals | | Mol File: | 191114-48-4.mol | 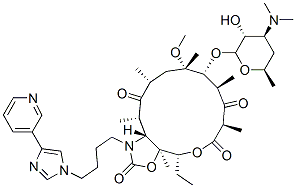 |
| | Telithromycin Chemical Properties |
| Melting point | 176-188 C | | Boiling point | 966℃ | | density | 1.26±0.1 g/cm3(Predicted) | | RTECS | KF4674500 | | Fp | >110°(230°F) | | storage temp. | Sealed in dry,Store in freezer, under -20°C | | solubility | Chloroform (Slightly), DMSO (Slightly), Methanol (Slightly) | | form | Solid | | pka | 10.85±0.70(Predicted) | | color | White to Light Beige | | Water Solubility | Sparingly soluble in water | | CAS DataBase Reference | 191114-48-4(CAS DataBase Reference) |
| | Telithromycin Usage And Synthesis |
| Macrolide antibiotics | Telithromycin is recently developed a new kind of macrolide antibiotics. It is the first antibiotic in the family of semi synthetic macrolides, a lincomycin B (MLSB) family. It is also the first ketone lactone antibiotic to be applied to the clinic. It is developed by the French Sanofi Aventis group successfully, with broad-spectrum antimicrobial activity and low resistance selection. It can be a good treatment of Streptococcus pneumoniae and resistant to penicillin and erythromycin, pneumococcal strains, Haemophilus influenzae bacteria and bacteria morahan, The antibacterial effect is stronger than the macrolide antibiotics such as azithromycin.It is mainly used for the treatment of respiratory tract infection, bronchitis, pharyngitis, tonsillitis and pneumonia.The function mechanism of Telithromycin is similar to the that of macrolide antibiotic.Mainly by binding to the 50S subunit of the bacterial ribosome, it inhibits the synthesis of protein and inhibits its translation and assembly. It can also be combined with nucleotides of the 23S ribosome RNA II and V structural region. But the biggest difference is that the binding force of telithromycin on wild-type ribosomes is about 10 times and 6 times stronger than that of erythromycin and clarithromycin. The slight difference in modification of ribosomal structure V region has made the MLSB antibiotic tolerance to bacteria increased by 20 times, making it effective for all drug-resistant strains of macrolide mg/(L•h). Oral administration absorbs well and its bioavailability is about 57%. Food does not affect its absorption. The 70% will be metabolized by CYP3A4 into theyrite alcohol, theyrite acid, N- deoxamide derivative and N- oxpyridine derivative in the liver. T1/2 is 9.81h, and the renal clearance rate was 12.5L/h. There are many ways of excreting: 13% are excreted from the urine in the original form, 3% are excreted from the feces in the original form , and 37% of the metabolites are excreted from the liver.In patients with liver dysfunction, Cmax decreases by about 20%, T1/2 is 1.4 times longer than normal people, and the metabolic rate decreases. The average pharmacokinetic parameters in patients with community acquired pneumonia (CAP) are Cmax 2.89 mg/L, Cmin 0.19 mg/L and AUC 13.9 mg/ (L. H).The US Food and Drug Administration announced on February 12, 2007It should make certain modifications to the drug labels of on the antibiotic telithromycin (also called Kenlike) produced by Sanofi Aventis. It will remove two items of acute bacterial sinusitis and chronic bronchitis in the indications of the drug from the drug instructions. It reminds patients to be careful in using telithromycin.
| | Description | Telithromycin was first launched in Germany as a once-daily oral treatment for
respiratory infections including community-acquired pneumonia, acute bacterial
exacerbations of chronic bronchitis, acute sinusitis and tonsillitis/pharyngitis. This
semisynthetic derivative of the natural macrolide erythromycin is the first marketed
ketolide, a new class of antibiotics featuring a C3-ketone instead of the L-cladinose group.
The 14-membered ring antibacterial agent prevents bacterial protein synthesis by binding
to two domains of the 50S subunit of bacterial ribosomes. It shows potent in vitro activity
against common respiratory pathogens including Streptococcus pneumoniae,
Haemophilus influenzae, Moraxella catarrhalis and Streptococcus pyogenes as well as
other atypical pathogens. The 3-keto group confers increased acidic stability and reduced
induction of macrolide-lincosamide-streptogramin B resistance that is frequently observed
with macrolides. The substituted C11-C12 carbamate residue appears not only to increase
affinity for the ribosomal binding site but also to stabilize the compound against esterase
hydrolysis and avoid resistance due to elimination of macrolides from the cell by an efflux
pump encoded by the mef gene in certain pathogens. Telithromycin is both a competitive
inhibitor and a substrate of CYP3A4. However, unlike several macrolides such as
troleandomycin, it does not form a stable inhibitory CYP P-450 Fe2+-nitrosoalkane
metabolite complex which is potentially hepatotoxic. The drug is well tolerated and well
distributed into pulmonary tissues, bronchial secretions, tonsils and saliva. It turns out to
be highly concentrated in azurophil granules of polymorphonuclear neutrophils thereby
facilitating its delivery to the phagocytosed bacteria. | | Chemical Properties | Pale Beige Solid | | Originator | Aventis (France) | | Uses | Telithromycin is a ketolide antibiotic, used to treat respiratory infections | | Uses | Telithromycin represents the first member of the current generation of erythromycin descendants, belonging to the ketolide class. The ketolides are characterised by the hydrolysis of the cladinose sugar and subsequent oxidation of the alcohol to a ketone. Telithromycin is acid stabile and has good activity against erythromycin-resistant S. aureus, and improved pharmacokinetics. | | Definition | ChEBI: Telithromycin is an amino sugar. | | Brand name | Ketek (Sanofi Aventis). | | Antimicrobial activity | The spectrum covers Gram-positive and Gramnegative cocci, Gram-positive bacilli, fastidious Gram-negative bacilli, atypical mycobacteria, M. leprae, H. pylori, anaerobes, T. pallidum, intracellular pathogens and atypical organisms.
It exhibits bactericidal activity in vitro against isolates of Str. pneumoniae regardless of the underlying resistance to penicillin G, erythromycin A and other agents. It is 2–4 times more active than clarithromycin against erythromycin A-susceptible isolates of Str. pneumoniae and other streptococci. Against H. influenzae the MIC range is 1–4 mg/L. It also exhibits good in-vitro activity against Coxiella burnetii (MIC 1 mg/L) and various Gram-positive species, including viridans streptococci (MIC ≤0.015–0.25 mg/L), C. diphtheriae (MIC 0.004–0.008 mg/L) and Listeria spp. (MIC 0.03–0.25 mg/L). | | Acquired resistance | It retains activity against isolates resistant to erythromycin A. Str. pneumoniae and Str. pyogenes isolates for which the MIC of telithromycin is above the resistance breakpoint of 2 mg/L are presently rare. It is not active against Staph. aureus isolates that owe their resistance to erythromycin to constitutive methylation of adenine 2058 on domain V of the peptidyl transferase loop. | | General Description | Telithromycin (Ketek) is an orally bioavailable macrolide.The antibiotic is semisynthetic. Telithromycin is classifiedas a ketolide, and it differs chemically from the macrolidegroup of antibacterials by the lack of α-L-cladinose at 3-position of the erythronolide A ring, resulting in a 3-ketofunction. It is further characterized by imidazolyl andpyridyl rings inked to the macrolide nucleus through a butylchain. The mechanism of action of telithromycin is the sameas that of the macrolide class.
Telithromycin causes a blockade of protein synthesis bybinding to domains II and V of 23S rRNA of the 50S ribosomalsubunit. Because telithromycin binds at domain II,activity against Gram-positive cocci is retained in the presenceof resistance mediated by methylases that alter thedomain V binding site. The antibiotic is also believed to inhibitthe assembly of ribosomes. Resistance to telithromycinoccurs because of riboprotein mutations. | | Pharmaceutical Applications | A 14-membered-ring ketolide, obtained by semisynthesis from erythromycin A. Formulated for oral administration. | | Pharmacokinetics | Oral absorption :90%
Cmax 800 mg oral :1.9–2.27 mg/L (steady state after 2–3 days)
Plasma half-life: 10–12 h
Volume of distribution :210 L
Plasma protein binding:60–70%
After oral administration the absolute bioavailability is 57% in both young and elderly subjects. The rate and extent of absorption are not influenced by food. In a study of ascending doses administered to healthy volunteers, peak plasma concentration ranged from 0.8 mg/L (400 mg dose) to 6 mg/L (2400 mg dose). The peak plasma concentration was reached after 1–2 h. The apparent elimination half-lives ranged from 10 to 14 h, with an AUC of 2.6 mg.h/L (400 mg dose) to 43.3 mg.h/L (2400 mg dose). After repeated oral doses the ratios between day 1 and day 10 ranged from 1.3 to 1.5. After once-daily oral dosing with 800 mg, the AUC is 8.25 mg.h/L. | | Side effects | It is generally well tolerated. The main adverse event is diarrhea. |
| | Telithromycin Preparation Products And Raw materials |
|