- acemetacin
-

- $66.00 / 1KG
-
2024-01-18
- CAS:53164-05-9
- Min. Order: 1250KG
- Purity: 99%
- Supply Ability: 330T
- Acemetacin
-

- $0.00 / 25KG
-
2023-08-18
- CAS:53164-05-9
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 50000KG/month
- Acemetacin
-
- $0.00 / 25kg
-
2023-04-04
- CAS:53164-05-9
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 1000kg
|
| | Acemetacin Basic information |
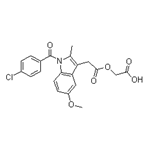
| Product Name: | Acemetacin | | Synonyms: | (1-(p-chlorbenzoyl)-5-methoxy-2-methylindol-3-acetoxy)essigsaeure;AURORA KA-6229;ACEMETACIN;[1-(4-CHLORO-BENZOYL)-5-METHOXY-2-METHYL-1H-INDOL-3-YL]-ACETIC ACID CARBOXYMETHYL ESTER;1-(4-Chlorobenzoyl)-5-methoxy-2-methyl-1H-indole-3-acetic Acid Carboxymethyl Ester;Emflex;Solart;1-(4-chlorobenzoyl)-5-methoxy-2-methyl-1h-indole-3-acetic aci carboxymethyl ((1-(4-chlorobenzoyl)-5-methoxy-2-methylindol-3-yl)acetoxy)acetic acid (1-(p-chlorbenzoyl)-5-methoxy-2-methylindol-3-acetoxy)essigsaeure 1-(4-chlorobenzoyl)-5-methoxy-2-methyl-1h-indole-3-acetic acid carboxymethyl 2-(2-(1-(p-chlorobenzoyl)-5-methoxy-2-methylindol-3-yl)acetoxy)acetic acid acemetacin acemetacinum acemix | | CAS: | 53164-05-9 | | MF: | C21H18ClNO6 | | MW: | 415.82 | | EINECS: | 258-403-4 | | Product Categories: | Intermediates & Fine Chemicals;Pharmaceuticals;EMFLEX;API | | Mol File: | 53164-05-9.mol | 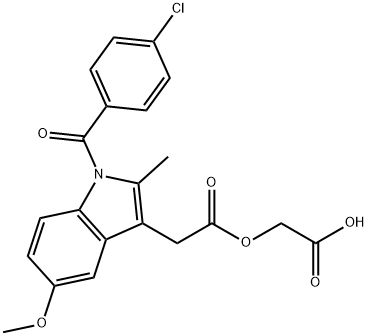 |
| | Acemetacin Chemical Properties |
| Melting point | 151.5°C | | Boiling point | 565.5±50.0 °C(Predicted) | | density | 1.2387 (rough estimate) | | refractive index | 1.6000 (estimate) | | RTECS | NL3521400 | | storage temp. | Sealed in dry,Store in freezer, under -20°C | | solubility | Practically insoluble in water, soluble in acetone, slightly soluble in anhydrous ethanol. | | pka | 2.60±0.10(Predicted) | | color | Very fine, pale-yellow crystals from pet eth | | Merck | 14,27 | | CAS DataBase Reference | 53164-05-9(CAS DataBase Reference) |
| Hazard Codes | T+ | | Risk Statements | 26/27/28 | | Safety Statements | 22-25-36/37/39-45-24/25 | | RIDADR | UN 2811 | | WGK Germany | 3 | | HazardClass | 6.1(a) | | PackingGroup | II | | HS Code | 29339900 | | Toxicity | LD50 in male, female mice, male, female rats (mg/kg): 55.5, 18.42, 24.2, 30.1 orally; 34.1, 51.1, 38.1, 28.3 i.v. (Jacobi, Dell) |
| | Acemetacin Usage And Synthesis |
| Description | Acemetacin is used in the treatment of pain
and restricted mobility resulting from chronic
articular rheumatism, degenerative articular disease, gout, and inflammation of muscle, joints,
and tendons. Acemetacin causes less gastrointestinal blood loss than indomethacin. Its anti-inflammatory activity results from liberation of
the parent compound, indomethacin. | | Chemical Properties | Light Yellow Solid | | Originator | Rantudil ,Bayer ,W. Germany ,1980 | | Uses | A potent inhibitor of COX-2 found to have anti-tumor activity in the colon | | Uses | Cyclo-oxygenase inhibitor; analgesic; anti-inflammatory. | | Definition | ChEBI: A carboxylic ester that is the carboxymethyl ester of indometacin. A non-steroidal anti-inflammatory drug, it is used in the treatment of rheumatoid arthritis, osteoarthritis, and low back pain, as well as for postoperative pain and inflammation. Its activ
ty is due to both acemetacin and its major metabolite, indometacin. | | Manufacturing Process | 25.4 g (0.050 mol) of [1-(p-chlorobenzoyl)-5-methoxy-2-methyl-3-
indoleacetoxy]-benzyl acetate were dissolved in 400 ml of glacial acetic acid
and hydrogenated on 2.0 g of palladium carbon at room temperature. After
the absorption of hydrogen had finished (1 hour), the catalyst was filtered off,
the filtrate was concentrated by evaporation under vacuum and the compound
was caused to crystallize by adding petroleum ether. The compound melted at
149.5-150.5°C (determined on the micro-Kofler bench); the yield was 19.4 g
which corresponds to 93% of the theoretical yield.
The starting material for the above step may be prepared as follows: 5 g
(0.016 mol) of N1-(p-methoxyphenyl)-p-chlorobenzhydrazide hydrochloride
and 4.75 g (0.018 mol) of benzyl levulinoyloxyacetate were heated in 25 ml of
glacial acetic acid for 3 hours at 80°C. The solvent was then evaporated off
under vacuum. The residue was taken up in chloroform and the solution was
washed neutral by shaking with sodium bicarbonate solution and thereafter
with water. After drying the chloroform solution, this was subjected to
chromatography on aluminium oxide, the eluate was concentrated by
evaporation and the viscous oil remaining as residue was crystallized by
adding ether. The compound melted at 94-95°C. The yield was 4.1 g which
corresponds to 50.7% of the theoretical yield. | | Therapeutic Function | Antiinflammatory | | Trade name | Acemetacin (Heumann, Stada,
Germany), Acemix (Bioprogress, Italy), Altren (Rh?one-Poulenc Rorer, Belgium), Emflex
(Merck, UK), Rantudil (Bayer Pharma Deutschland, Germany) | | Clinical Use | Acemetacin is a nonsteroidal
anti-inflammatory drug acting directly
and via its major metabolite indomethacin.
Acemetacin is used in chronic joint pain as well
as in postoperative pain. | | Safety Profile | Poison by ingestion, subcutaneous,intraperitoneal, intravenous, and intramuscular routes. Anexperimental teratogen. Other experimental reproductiveeffects. When heated to decomposition it emits toxic fumesof Cl?? and NOx. An anti-inflammatory agent. | | Synthesis | alkylation of indomethacin with
benzyl bromoacetate in K2CO3/N,N-dimethylformamide gives the corresponding benzyl glycolate ester, which is hydrogenated over
10 % palladium on charcoal in acetic acid to
yield acemetacin. |
| | Acemetacin Preparation Products And Raw materials |
|