西立伐他汀钠
| 中文名称 | 西立伐他汀钠 |
|---|---|
| 中文同义词 | (+)-(3R,5S,6E)-7-[4-(4-氟苯基)-2,6-二异丙基-5-甲氧甲基-吡啶-3-基]-3,5-二羟基-6-庚烯酸单钠盐;西伐他汀;西立伐他汀;化合物 T14931 |
| 英文名称 | CERIVASTATIN SODIUM |
| 英文同义词 | RIVASTATIN;sodium 7-[4-(4-fluorophenyl)-5-(methoxymethyl)-2,6-dipropan-2-yl-pyrid in-3-yl]-3,5-dihydroxy-hept-6-enoate;LIPOBAY;BAYCOL;CERIVASTATIN NA;CERIVASTATIN, SODIUM SALT;7-[4-(4-fluorophenyl)-5-(methoxymethyl)-2,6-dipropan-2-yl-pyrid in-3-yl]-3,5-dihydroxy-hept-6-enoate;(3R,5S,6E)-7-[4-(p-Fluorophenyl)-2,6-diisopropyl-5-(methoxymethyl)-3-pyridyl]-3,5-dihydroxy-6-heptenoic acid |
| CAS号 | 145599-86-6 |
| 分子式 | C26H34FNO5 |
| 分子量 | 459.55 |
| EINECS号 | |
| 相关类别 | Active Pharmaceutical Ingredients |
| Mol文件 | 145599-86-6.mol |
| 结构式 | 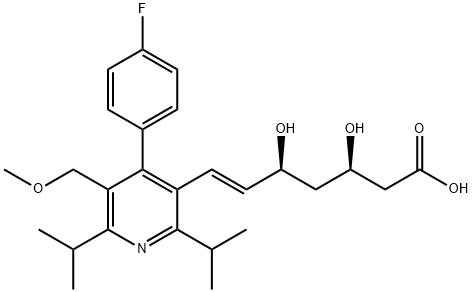 |
西立伐他汀钠 性质
| 熔点 | >176oC (dec.) |
|---|---|
| 沸点 | 646.3±55.0 °C(Predicted) |
| 密度 | 1.181±0.06 g/cm3(Predicted) |
| 储存条件 | Hygroscopic, -20°C Freezer, Under Inert Atmosphere |
| 溶解度 | 乙醇(微溶)、甲醇(微溶)、水(微溶) |
| 形态 | 固体 |
| 酸度系数(pKa) | pKa 4.38 (H2O t=25 I=0.025) (Uncertain);5.29(H2O t=25 I=0.025) (Uncertain) |
| 颜色 | 白色至类白色 |
| 稳定性 | 吸湿性 |
| CAS 数据库 | 145599-86-6(CAS DataBase Reference) |
| EPA化学物质信息 | 6-Heptenoic acid, 7-[4-(4-fluorophenyl)-5-(methoxymethyl)-2,6-bis(1- methylethyl)-3-pyridinyl]-3,5-dihydroxy-, (3R,5S,6E)- (145599-86-6) |
西立伐他汀口服后吸收迅速而完全由于药物和肝脏有高度亲和力,所以大部分药物停留在肝脏发挥作用,肝脏首次关卡效应低。西立伐他汀的生物转化主要发生在肝脏,药物主要转化为M1,M23和M24,且均有生理活性。一次口服西立伐他汀0.2mg,Cmax为2.98μg•L-1,Tmax为1~4h,AUC0~∞为14.19μg•L-1•h-1,T12为3.26h。药物动力学参数性别间无明显差别[6]。34%药物从肾脏排泄,其余药物经肠肝循环排泄。轻度肾功能不全时不需调整用药剂量。西立伐他汀的一个显著优点是与其他药物(如华法令、地高辛)在药物动力学方面无相互干扰。
西立伐他汀具有很好的调脂作用。观察到西立伐他汀0.2mg•d-1应用5wk后可使血清低密度脂蛋白胆固醇(LDL-C)降低30.5%。与此同时西立伐他汀可降低血清TC,TG,载脂蛋白B,升高血清高密度脂蛋白胆固醇(HDL-C)浓度。西立伐他汀0.025~0.4mg•d-1可使血清LDL-C明显下降,下降幅度与药物剂量成正相关。当西立伐他汀剂量为0.4mg•d-1时,大于40%的病人血清LDL-C下降大于40%,9%病人血清LDL-C下降大于50%。在该剂量下血清TG明显下降,而HDL-C、载脂蛋白 A-1明显上升。对2593例使用西立伐他汀的病人进行分析,对照组(520例)不用药。疗效统计均取8wk。
Cerivastatin 是一种合成的降脂剂,是一种高效,耐受性好,口服活性的 HMG-CoA 还原酶抑制剂,Ki 为 1.3 nM/L。Cerivastatin 可降低低密度脂蛋白胆固醇水平。Cerivastatin 还主要通过 RhoA 抑制作用来抑制 MDA-MB-231 细胞的增殖和侵袭,具有抗癌作用。Ki: 1.3 nM/L (HMG-CoA reductase)
Cerivastatin (5-50 ng/mL; 3 days; MDA-MB-231 cells) treatment induces a dose-dependent decrease in cell proliferation of MDA-MB-231 cells (up to 40% inhibition at 25 ng/mL).
Cerivastatin (25 ng/mL; 18-36 hours; MDA-MB-231 cells) treatment induces an arrest of the cell cycle in G 1/S phase after 36 h treatment. This arrest is not observed for a shorter incubation time (18 h).
Cerivastatin (25 ng/mL; 18 hours; MDA-MB-231 cells) treatment induces a marked increase in the level of p21
Waf1/Cip1
.
Cerivastatin (25 ng/mL; 12 hours; MDA-MB-231 cells) treatment increases the p21 transcript in MDA-MB-231 cells.
Cerivastatin (10-25 ng/mL; 18 hours) inhibits invasion of MDA-MB-231 cells through Matrigel.
Cerivastatin (25 ng/mL; 18-36 hours) delocalizes RhoA and Ras from the membrane to the cytosol and induces morphological changes.
Cerivastatin (25 ng/mL; 4-36 hours) induces inactivation of NFκB, in a RhoA inhibition-dependent manner, resulting in a decrease in urokinase and metalloproteinase-9 expression, and concomitantly increases IκB.
Cell Proliferation Assay
| Cell Line: | MDA-MB-231 cells |
| Concentration: | 5 ng/mL, 10 ng/mL, 25 ng/mL, 50 ng/mL |
| Incubation Time: | 3 days |
| Result: | Induced a dose-dependent decrease in cell proliferation of MDA-MB-231 cells. |
Cell Cycle Analysis
| Cell Line: | MDA-MB-231 cells |
| Concentration: | 25 ng/mL |
| Incubation Time: | 18 hours, 36 hours |
| Result: | Induced a cell cycle block in G 1/S phase. |
Western Blot Analysis
| Cell Line: | MDA-MB-231 cells |
| Concentration: | 25 ng/mL |
| Incubation Time: | 18 hours |
| Result: | Induced a marked increase in the level of p21 Waf1/Cip1 . |
RT-PCR
| Cell Line: | MDA-MB-231 cells |
| Concentration: | 25 ng/mL |
| Incubation Time: | 12 hours |
| Result: | Increased p21 Waf1/Cip1 mRNA levels. |
Cerivastatin is well absorbed, reaching maximal plasma levels in 1-3 hours following oral dosing. In the circulation, Cerivastatin is highly bound to plasma proteins (99.5%), with an elimination half-life of 2-4 hours. Cerivastatin is metabolized predominantly in the liver to three polar metabolites. Two of these metabolites are active, but to a lesser extent compared to parent drug, and the third metabolite is inactive. Plasma concentrations of all metabolites are substantially lower than those of the parent drug. Elimination of metabolites is via the urine (20-25%) and feces (66-73%), while essentially no parent compound is excreted.
