- Atropine
-

- $50.00 / 1kg
-
2023-04-18
- CAS:51-55-8
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 100 tons
- Atropine
-

- $0.00 / 1Kg/Bag
-
2021-09-29
- CAS:51-55-8
- Min. Order: 1KG
- Purity: 99.0% ~ 100.5%,USP30
- Supply Ability: 200kg/month
Related articles - Uses of Atropine
- Atropine is the racemic mixture of L- and D-hyoscyamine and possesses 50% of the antimuscarinic potency of L-hyoscyamine. Atro....
- Nov 16,2021
|
| Product Name: | Atropine | | Synonyms: | 1alphaH,5alphaH-Tropan-3alpha-ol (.+/-.)-tropate (ester);1-alpha-h,5-alpha-h-tropan-3-alpha-ol(+-)-tropate(ester);1alphah,5alphah-tropan-3alpha-ol(+-)-tropate(ester);2-Phenylhydracrylic acid 3-alpha-tropanyl ester;2-phenylhydracrylicacid3-alpha-tropanylester;alpha-(Hydroxymethyl)benzeneacetic acid 8-methyl-8-azabicyclo(3.2.1)oct-3-yl ester;alpha-(hydroxymethyl)benzeneaceticacid8-methyl-8-azabicyclo(3.2.1)oct-3-yl;Atropina | | CAS: | 51-55-8 | | MF: | C17H23NO3 | | MW: | 289.37 | | EINECS: | 200-104-8 | | Product Categories: | Pharmaceutical;Inhibitors;ATROPISOL;Alkaloids;Biochemistry;Tropane Alkaloids | | Mol File: | 51-55-8.mol | 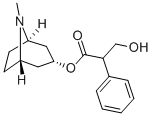 |
| | Atropine Chemical Properties |
| Melting point | 115-118 °C | | Boiling point | 431.53°C (rough estimate) | | density | 1.0470 (rough estimate) | | refractive index | 1.5200 (estimate) | | Fp | 2℃ | | storage temp. | -20°C | | solubility | H2O: 2 mg/mL | | form | powder | | pka | 9.7(at 21℃) | | color | white | | Water Solubility | 1.6g/L(18 ºC) | | Sensitive | Light Sensitive | | Merck | 14,875 | | BRN | 91260 | | InChIKey | RKUNBYITZUJHSG-SPUOUPEWSA-N | | CAS DataBase Reference | 51-55-8(CAS DataBase Reference) | | NIST Chemistry Reference | Atropine(51-55-8) | | EPA Substance Registry System | Atropine (51-55-8) |
| | Atropine Usage And Synthesis |
| Overview | Atropine is an alkaloid extracted from the Atropa bell-adonna, Datura stramonium, or Hyosc-yamus niger. What is naturally occurring is the unstable L-hyoscyamine, which can be converted to the stable racemate atropine after chemical treatment. It can also be chemically synthesized; what is commonly used in its sulfate. It appears as white crystalline powder, being odorless, bitter, and soluble in water and alcohol. Upon contact with alkaline drugs, it can cause decomposition. Atropine blocks the M-choline receptor, thereby antagonizing the M-like effect of acetylcholine or its quasi-drug (muscarinic effect).
The main effect is as follows:
- Relax the smooth muscle: This product has the role of relaxation of many visceral smooth muscle; it has remarkable relaxation effect on over-activity or spammed smooth muscle, while having less effect on the normal activity of the smooth muscle.
- Inhibition of glandular secretion: inhibit the glandular secretion by blocking the M-choline receptor. The most obvious effect occurs on salivary glands and sweat glands. It can also greatly reduce the secretion of lacrimal glands and respiratory glands, but the impact on gastric acid secretion is smaller.
- The effect on the eye: atropine, through blocking the pupil sphincter and ciliary muscle M-choline receptors, is manifested as mydriasis, increased intraocular pressure and adjust paralysis. All three effects have important clinical implications.
- The effect on the cardiovascular: a larger dose (1 ~ 2mg) atropine can get rid of the inhibitory effect of the vagus nerve on the heart, thus accelerating the heart rate. Large doses can dilate the skin and visceral blood vessels, and relieve arteriolar spasm. Its mechanism of dilating blood vessels and relieving small vasospasm is unknown.
- Excitement of the central nervous system: high-dose cause irritability, prolonged talk and delirium. Poisoning dose (more than 10mg) can produce hallucinations, disorientation, involuntary exercise and convulsions.
| | Chemical properties | It appears as white crystalline powder, odorless, tasteless. Mp 114-116°C; sublimation at 93-110 °C under high vacuum. The solubility of this product in each solvent: 80 ℃ hot water (1:90), ethanol (1: 2), chloroform (1: 1), ether (1:25), dissolved in benzene and dilute acid solution. Low toxicity, LD50 (rat, oral): 622mg / kg. | | Pharmacokinetics | It can be quickly absorbed by the gastrointestinal tract after oral administration and quickly distributed to the body tissue. It can also penetrate through the placenta into the fetal circulation. Clinical studies have shown that after intramuscular injection of 2 mg, 85 ~ 88% is subject to urine excretion within 24 hours. Approximately 5% of them were found in their prototypes and 33% of their metabolites; only a small amount was excreted in feces and other secretions. | | Side effects | Dry mouth, blurred vision, heart palpitations, dry skin and constipation. High-dose poisoning switches excitement into inhibition, causing coma and respiratory paralysis and death.
| | Production | It can be extracted from the root of Solanaceae Scoparia Anisodus tanguticus or Himalayan Scoparia; can also be obtained by artificial synthesis.
Belladonna leaf is extracted out of the scopolamine (L-body), followed by racemization, recrystallization to make it.
| | Category | toxic substances | | Toxicity grade | highly toxic | | Acute Toxicity | Oral - Rat LD50: 500 mg / kg; Oral - Mouse LD50: 75 mg / kg | | Flammability Hazard characteristics | Flammable; Combustion produces toxic nitrogen oxide fumes; Patient medication side effects: Visual changes; Dilated pupils, Muscle weakness.
| | Storage and transportation | Ventilated, dry and low temperature; Separated from foodstuffs in storehouse | | Extinguishing agent | dry powder, foam, sand, carbon dioxide, water mist | | Description | Atropine is a naturally occurring tropane alkaloid extracted from plants of the family Solanaceae including deadly nightshade (A. belladonna). It is a non-selective, competitive antagonist of the muscarinic acetylcholine receptor types M1, M2, M3, M4, and M5 (pKBs range from 8.9-9.8). Atropine increases firing of the sinoatrial node and conduction through the atrioventricular node of the heart, opposes the actions of the vagus nerve, blocks acetylcholine receptor sites, and decreases bronchial secretions. It is classified as an anticholinergic (parasympatholytic) drug and commonly used to dilate the pupils, increase heart rate, reduce salivation and other secretions, and as an antidote against organophosphate poisoning. | | Description | Atropine is considered to be the
most effective antidote for both OP and CB intoxication.
By effectively competing with acetylcholine for the
same cellular receptors, it prevents overstimulation of
the autonomous parasympathetic system. Most importantly,
it helps prevent asphixia, the main cause of
death. In human subjects, it is customary to constantly
infuse atropine in order to maintain optimal concentration
throughout recovery from the “cholinergic crisis.” In
wildlife rehabilitation, this is impractical and subjects
need to be repeatedly injected with atropine. | | Chemical Properties | Atropine, also known as daturine, C17H23NO3, white, crystalline substance, optically inactive, but usually contains levorotatory hyoscyamine. Compound is soluble in alcohol, ether, chloroform, and glycerol; slightly soluble in water. | | Chemical Properties | White or almost white, crystalline powder or colourless crystals. | | Physical properties | Appearance: atropine appears as colourless, odourless crystals or a white crystalline
powder. Solubility: very soluble in water and soluble in ethanol. Melting point:
melting point of atropine isn’t higher than 189?°C (melting time decomposition)
(Chinese Pharmacopoeia), 114–118?°C (United States Pharmacopeia) and 115–
119?°C (British Pharmacopoeia). The chemical structure of atropine is made up of
amino alcohol esters. It is easy for atropine to be hydrolysed into tropine and despun
tropic acid under alkaline condition. Atropine is stable in faintly acid and neutral
aqueous solution, most stable at pH 3.5–4.0. | | Originator | Atromed,Promed Exports,India | | History | Mandragora (mandrake) was described for treatment of wounds, gout and sleeplessness and as a love potion in the fourth century BC by Theophrastus. Atropine
extracted from the Egyptian henbane was used by Cleopatra in the last century BC
to dilate her pupils in the hope that she would appear more alluring. In the
Renaissance, women used the juice of the berries of Atropa belladonna to enlarge
the pupils of their eyes for cosmetic reasons.
It isn’t until the first century AD that Dioscorides found that wine containing
mandrake can be used as an anaesthetic treatment for pain or sleeplessness in surgery or cautery. The combination of extracts containing tropane alkaloids and opium
was used to treat diseases, which was popular in the Roman and Islamic Empires
and Europe. The combination was replaced by the use of ether, chloroform and
other modern anaesthetics about 100?years ago.
The mydriatic effects of atropine were studied by the German chemist Friedlieb
Ferdinand Runge (1795–1867). In 1831, the German pharmacist Heinrich F.? G.
Mein (1799–1864) succeeded in separating pure atropine from plants. The substance was first synthesized by German chemist Richard Willst?tter in 1901.
In 1889, Richard Willst?tter first confirmed the chemical structure of atropine.
Atropine was first synthesized by A.?Ladenburg. Homatropine, a kind of tropic alkaline ester, is used in the diagnosis and treatment in ophthalmology, and it has a
shorter acting time than atropine. Quaternary ammonium compounds of atropine
obtained by alkylation of nitrogen atoms have anticonvulsant function, which does
not affect the central nervous system, due to their polarity. In 1970, atropine sulphate was synthesized in Hangzhou, the location of the first pharmaceutical factory
in China, which increased the yield, reduced the cost and met the requirements of
clinics. | | Uses | Scopolamine is found in the leaves of Daturametel L., D. meteloides L., and D. fastuosavar. alba (Cordell 1978). It is used as asedative, a preanesthetic agent, and in thetreatment of motion sickness (Merck 1989). | | Uses | anticholinergic, mydriatic | | Uses | Atropine is used in medicine and is an antidote for cholinesteraseinhibiting compounds, such as organophosphorus insecticides and certain nerve gases. Atropine is commonly offered as the sulfate. Atropine is used in connection with the treatment of disturbances of cardiac rhythm and conductance, notably in the therapy of sinus bradycardia and sick sinus syndrome. Atropine is also used in some cases of heart block. In particularly high doses, atropine may induce ventricular tachycardia in an ischemic myocardium. Atropine is frequently one of several components in brand name prescription drugs. | | Production Methods | Atropine is prepared by extraction from Datura stramonium, or synthesized. The compound is toxic and allergenic. | | Definition | atropine: A poisonous crystalline alkaloid,C17H23NO3; m.p. 118–119°C. Itcan be extracted from deadly nightshadeand other solanaceous plantsand is used in medicine to treat colic,to reduce secretions, and to dilatethe pupil of the eye. | | Definition | An alkaloid that is the 3(s)-endo isomer of atropine. | | Indications | This product was recorded in the Pharmacopoeia of the People’s Republic of China
(2015), the British Pharmacopoeia (2017), the United States Pharmacopeia (40), the
Japanese Pharmacopoeia (17th ed.), the Indian Pharmacopoeia (2010), the European
Pharmacopoeia (9.0th ed.), the International Pharmacopoeia (5th ed.) and the
Korean Pharmacopoeia (10th ed.).
Atropine sulphate is commonly used in clinics. Dosage forms are injection, tablet and eye ointment; atropine sulphate was mainly used to treat toxic shock and
organic phosphorus pesticide poisoning, to relieve visceral colic, as preanaesthetic
medication and to reduce bronchial mucus secretion. The indications of atropine
sulphate eye gel are iridocyclitis, fundus examination and mydriasis. | | Manufacturing Process | Atropin was obtained from belladonna roots and by racemisation of Lhyoscyamine
with dilute alkali or by heating in chloroform solution. The
alkaloid was crystallised from alcohol on addition of water, or from chloroform
on addition of light petroleum, or from acetone in long prisms, m.p. 118°C,
sublimed unchanged when heated rapidly. It is soluble in alcohol or
chloroform, less soluble in ether or hot water, sparingly so in cold water (in
450 L at 25°C) and almost insoluble in light petroleum. Atropine is optically
inactive. | | Brand name | Atrophate [Veterinary]
(Schering-Plough Animal Health); Atropisol
(Ciba Vision, US Ophthalmics); Isopto Atropine (Alcon). | | Therapeutic Function | Anticholinergic | | World Health Organization (WHO) | Atropine, an alkaloid with anticholinergic activity extracted from
Atropa belladonna, has been widely used in medicines for centuries for its
antispasmodic and mydriatic properties. It is also used for premedication prior to
anaesthesia. Preparations containing atropine remain available and the substance
is included in the WHO Model List of Essential Drugs. | | General Description | Atropine is the tropine ester of racemictropic acid and is optically inactive. It possibly occurs naturallyin various Solanaceae, although some claim, with justification,that whatever atropine is isolated from naturalsources results from racemization of (-)-hyoscyamine duringthe isolation process. Conventional methods of alkaloidisolation are used to obtain a crude mixture of atropine andhyoscyamine from the plant material. This crude mixture isracemized to atropine by refluxing in chloroform or by treatmentwith cold dilute alkali. Because the racemizationprocess makes atropine, an official limit is set on thehyoscyamine content by restricting atropine to a maximumlevorotation under specified conditions.
Atropine occurs in the form of optically inactive, white,odorless crystals possessing a bitter taste. It is not very solublein water (1:460, 1:90 at 80°C) but is more soluble inalcohol (1:2, 1:1.2 at 60°C). It is soluble in glycerin (1:27),in chloroform (1:1), and in ether (1:25). Saturated aqueoussolutions are alkaline in reaction (pH 9.5). The free baseis useful when nonaqueous solutions are to be made, such asin oily vehicles and ointment bases. Atropine has a plasmahalf-life of about 2 to 3 hours. It is metabolized in the liverto several products, including tropic acid and tropine. | | Hazard | Extremely toxic, poison, paralyzes the
parasympathetic nervous system by blocking the
action of acetylcholine at nerve endings. | | Health Hazard | The toxic effects are similar to atropine. Thesymptoms at toxic doses are dilation of the pupils, palpitation, blurred vision, irritation,confusion, distorted perceptions, hallucinations,and delirium. However, the mydriaticeffect is stronger than that of many othertropane alkaloids. Scopolamine is about threeand five times more active than hyocyamineand atropine, respectively, in causing dilationof the pupils. Its stimulating effect on thecentral nervous system, however, is weakerthan that of cocaine but greater than thatof atropine. The oral LD50 value in mice iswithin the range of 1200 mg/kg.
The histidine reversion–Ames test formutagenicity gave inconclusive results. | | Pharmacology | Atropine is a blocker of typical M-choline receptor. In addition to terminating the
gastrointestinal smooth muscle spasm, inhibiting glands, dilating pupils, increasing
intraocular tension, adjusting vision through paralysis, accelerating heart rate and
dilating bronchi, large doses of atropine dilate blood vessels, terminating the spasmodic contraction and improving minicirculation. Atropine can excite or inhibit the
central nervous system in a dose-dependent manner. Atropine exerts longer and
stronger effect on heart, intestine and bronchial smooth muscle than other belladonna alkaloids. Atropine also relaxes the pupillary sphincter and the ciliary muscle
and dilates the pupils by blocking M-choline receptor in ocular tissue.
Blockers of M-choline receptor included atropine, scopolamine, anisodamine
and anisodine. Belladonnas not only block M-choline receptor in internal organ
cells but also in the central nervous system. Compared with atropine, scopolamine
has an oxygen bridge, which increases central nervous system function. The oxygen
bridge of scopolamine is partially broken and then becomes anisodamine, which
is difficult to pass through the blood-brain barrier, and symptoms caused by atropine
in the central nervous system were less than that caused by atropine.
Peak concentration of plasma can be reached at 15–20?min after intramuscular
injection of atropine and at 1–2?h after oral administration and can last for 4–6?h.
Most of the atropine can be absorbed by the gastrointestinal tract and other mucous
membranes, and a little of the atropine can be absorbed by the eyes and skin. The t1/2
is 3.7–4.3?h. Binding rate of plasma protein is 14–22%. Volume of distribution is
1.7?L/kg after oral administration. Atropine can rapidly distribute to different organ
systems and pass the blood-brain barrier and the placenta.
After absorption by the eye’s conjunctiva, 30% of the products are excreted
unchanged via the kidneys; the others become metabolites by hydrolysis and glucuronidation or glucosidation. After 1% gel eye drop, enlarged pupil function lasts
for 7–10?days, and regulatory paralysis lasts for 7–12?days. | | Clinical Use | The best known of the muscarinic blocking drugs are the
belladonna alkaloids, atropine (Atropine) and scopolamine
(Scopolamine).They are tertiary amines that contain
an ester linkage. Atropine is a racemic mixture of
DL-hyoscyamine, of which only the levorotatory isomer is
pharmacologically active.Atropine and scopolamine are
parent compounds for several semisynthetic derivatives,
and some synthetic compounds with little structural similarity
to the belladonna alkaloids are also in use.All of
the antimuscarinic compounds are amino alcohol esters
with a tertiary amine or quaternary ammonium group. | | Safety Profile | Poison by ingestion,
subcutaneous, intravenous, and
intraperitoneal routes. Human systemic
effects by ingestion and intramuscular
routes: visual field changes, mydriasis
@updlary dtlation), and muscle weakness.
An experimental teratogen. Other
experimental reproductive effects. An
alkaloid. When heated to decomposition it
emits toxic fumes of NOx. | | Synthesis | Atropine, the D,L-8-methyl-8-azabicyclo[3.2.1]oct-3-yl ester of |á-hydroxymethyl
phenylacetic acid (14.1.4), can be synthesized by a standard scheme of synthesizing of
tropane alkaloids. Condensation of maleyl aldehyde with methylamine and acetonedicar�boxylic acid gives tropenone (14.1.1), which is the main starting material for the synthesis of
both atropine and scopolamine. The carbonyl group of tropenone is reduced, thus forming
tropenol (14.1.2), after which the double bond between C6 and C7 of the tropane ring is
hydrogenated, giving tropine (14.1.3). Esterification of the tropenol gives the desired
atropine (14.1.4) [1¨C6]. 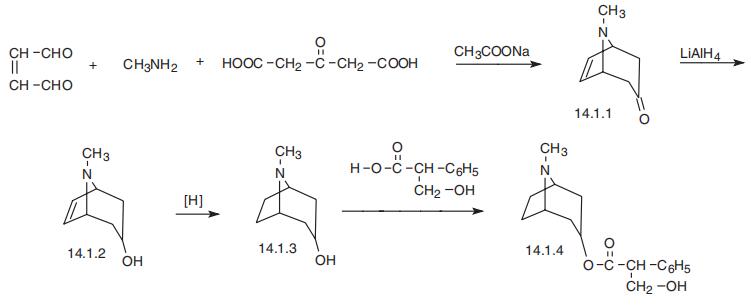
| | Environmental Fate | Atropine competitively antagonizes acetylcholine at the neuroreceptor
site. Atropine prevents acetylcholine from exhibiting
its usual action but does not decrease acetylcholine production.
Cardiac muscle, smooth muscle, and the central nervous
system are most affected by the antagonism of acetylcholine. | | Purification Methods | Atropine crystallises from acetone or hot water, and sublimes at ~ 100o/high vacuum. [Beilstein 21/1 V 235.] | | Toxicity evaluation | Free atropine is only slightly soluble in cold water. It melts at
115°C but decomposes upon boiling.
Environmental monitoring of atropine is not routinely
performed by regulatory bodies. Hazardous short-term degradation
products are not likely to occur. Accidental environmental
exposure may occur through unintentional ingestion of
toxic plants of the Solanaceae family, such as the deadly
nightshade. |
| | Atropine Preparation Products And Raw materials |
|