- Meloxicam
-

- $0.00 / 1kg
-
2024-04-07
- CAS:71125-38-7
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 1000kg
- Meloxicam
-

- $35.00/ kg
-
2024-03-16
- CAS:71125-38-7
- Min. Order: 1kg
- Purity: 99.8%
- Supply Ability: 200tons/year
- Meloxicam
-

- $0.00 / 1KG
-
2024-03-16
- CAS:71125-38-7
- Min. Order: 100g
- Purity: 98%+
- Supply Ability: 100kg
|
| | Meloxicam Basic information |
| Product Name: | Meloxicam | | Synonyms: | MeloxicaM, BP;MeloxicaM API;4-Hydroxy-2-Methyl-N-(5-Methylthiazol-2-yl)-2H-benzo[e][1,2]thiazine-3-carboxaMide 1,1-dioxide;Meloxicam Solution, 100ppm;4-hydroxy-2-methyl-N-(5-methyl-1,3-thiazol-2-yl)-2H-1,2-benzothiazine-3-carboxamide 1,1-dioxide;4-Hydroxy-2-methyl-N-(5-methyl-1,3-thiazol-2-yl)-2H-1,2-benzothiazine-3-carboxamide 1,1-dioxid;Coxicam;MOBEC | | CAS: | 71125-38-7 | | MF: | C14H13N3O4S2 | | MW: | 351.4 | | EINECS: | 615-253-8 | | Product Categories: | Other APIs;AVAPRO;Lipid signaling;API's;Active Pharmaceutical Ingredients;API;Heterocycles;Intermediates & Fine Chemicals;Pharmaceuticals;Sulfur & Selenium Compounds;Health & Beauty;71125-38-7 | | Mol File: | 71125-38-7.mol | 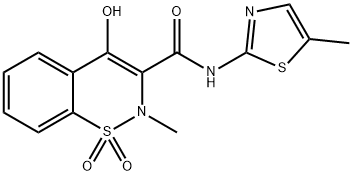 |
| | Meloxicam Chemical Properties |
| Melting point | 255 °C | | density | 1.613±0.06 g/cm3(Predicted) | | storage temp. | 2-8°C | | solubility | DMSO: soluble | | form | Off-white solid | | pka | 4.08 in water; 4.24 ± 0.01 in water/ethanol (1:1); 4.63 ± 0.03 in water/ethanol (1:4) | | color | yellow | | Water Solubility | practically insoluble in water(0.154 mg/mL), with higher solubility observed in strong acids and bases. | | Merck | 14,5826 | | BCS Class | 2(CLogP),
4 (LogP) | | Stability: | Stable. Incompatible with strong oxidizing agents. | | InChI | InChI=1S/C14H13N3O4S2/c1-8-7-15-14(22-8)16-13(19)11-12(18)9-5-3-4-6-10(9)23(20,21)17(11)2/h3-7,18H,1-2H3,(H,15,16,19) | | InChIKey | ZRVUJXDFFKFLMG-UHFFFAOYSA-N | | SMILES | S1(=O)(=O)C2=CC=CC=C2C(O)=C(C(NC2=NC=C(C)S2)=O)N1C | | EPA Substance Registry System | 2H-1,2-Benzothiazine-3-carboxamide, 4-hydroxy-2-methyl-N-(5-methyl-2-thiazolyl)-, 1,1-dioxide (71125-38-7) |
| | Meloxicam Usage And Synthesis |
| Description | Known as a nonsteroidal anti-inflammatory drug (NSAID), Meloxicam is commonly used to treat pain or inflammation caused by rheumatoid arthritis and osteoarthritis in adults. It is also used to treat juvenile rheumatoid arthritis in children who are at least 2 years old. It is effective to reduce pain, inflammation, swelling, and stiffness of the joints. Developed by Boehringer-Ingelheim, Meloxicam is a derivative of oxicam that can relieve the symptoms of arthritis, primary dysmenorrhea, fever with analgesic and antipyretic properties. Meloxicam was approved for use in April 2000.
Anti-inflammatory effects of meloxicam function by inhibiting the prostaglandin synthetase (cyclooxygenase 1 and 2) which results in a decrease of the synthesis of prostaglandins. As prostaglandins are chemicals that contribute to inflammation especially within joints, which leads to the common symptoms of pain, tenderness, and swelling associated with arthritis, inhibition of their synthesis can be associated with the analgesic and antipyretic effects of meloxicam. As a result, inflammation and its accompanying symptoms are reduced. Meloxicam starts to relieve pain about 30 to 60 minutes after administration. | | Chemical Properties | Meloxicam is a light yellow solid, practically insoluble in water, with higher solubility observed in strong acids and bases. It is very slightly soluble in methanol. Meloxicam has an apparent partition coefficient (log P)app = 0.1 in n-octanol/buffer pH 7.4. It has pKa values of 1.1 and 4.2. | | Originator | Boehringer Ingelheim (Germany) | | Uses | Meloxicam is a preferential cyclooxygenase (COX-2) inhibitor that used for symptomatic treatment of arthritis and osteoarthritis. Sudoxicam and Meloxicam are nonsteroidal anti-inflammatory drugs (NSAIDs) from the enol-carboxamide class. | | Uses | An inhibitor of Cox-1 and Cox-2, selective for Cox-2. | | Indications | Meloxicam (Mobic), recently introduced for the
treatment of osteoarthritis, is also used for rheumatoid
arthritis and certain acute conditions. Although meloxicam
is sometimes reported to be a selective COX-2 inhibitor,
it is considerably less selective than celecoxib or
rofecoxib. Its adverse effects are similar to those of
piroxicam and other NSAIDs; however, the frequency
of GI side effects is lower for meloxicam than for piroxicam
and several other NSAIDs. | | Definition | ChEBI: Meloxicam is a benzothiazine that is piroxicam in which the pyridin-2-yl group is replaced by a 5-methyl-1,3-thiazol-2-yl group. A non-steroidal anti-inflammatory drug and selective inhibitor of COX-2, it is used particularly for the management of rheumatoid arthritis. It has a role as a non-steroidal anti-inflammatory drug, an antirheumatic drug, a cyclooxygenase 2 inhibitor and an analgesic. It is a benzothiazine, a monocarboxylic acid amide and a member of 1,3-thiazoles. | | Manufacturing Process | A mixture of 26.9 g (0.1 mol) of the 1,1-dioxide of methyl 4-hydroxy-2- methyl-2H-1,2-benzothiazine-3-carboxylate and 12.5 g (0.11 mol) of 2- amino-5-methylthiazole was refluxed in 4 liters of xylene for 24 hours in a nitrogen atmosphere. The methanol formed by the reaction was removed by means of a 4-A-molecular sieve mounted in a Soxhlet-extractor. The hot reaction solution was filtered. Upon cooling and standing overnight, the crude product separated out of the filtrate in the form of crystals (32.0 g, 91% of theory). After recrystallization from ethylene chloride 26.0 g (74% of theory) of 4-hydroxy-2-methyl-N-(5-methyl-2-thiazolyl)-2H-1,2-benzothiazine-3- carboxamide-1,1-dioxide were obtained; M.P.: 254°C (decomp.). | | Brand name | Mobic
(Boehringer Ingelheim). | | Therapeutic Function | Antiinflammatory | | General Description | Meloxicam (Mobic) is a selective COX-2 inhibitor amongoxicams indicated for use in RA and OA. It also has a relativelylong half-life of 15 to 20 hours and has a much lowerrate of serious GI side effects and a lower than average riskof nephropathy when compared with other conventionalNSAIDs.196 The recommended dose is 7.5 mg once dailywith a maximum of 15 mg/d. Meloxicam is metabolized inhumans mainly by CYP2C9 (with a minor contribution viaCYP3A4) to 5'-hydroxymethylmeloxicam and 5 carboxymeloxicam.
In large-scale comparative trials, meloxicam was foundto be at least as effective as most conventional NSAIDs inthe treatment of rheumatic disease or postoperative pain, buthas demonstrated a more favorable GI tolerability profile. | | Biological Activity | Meloxicam is a selective inhibitor of COX-2 (IC50s = 11.8 and 143 μM for COX-2 and COX-1, respectively, in an enzyme activity assay) and a non-steroidal anti-inflammatory drug (NSAID). Meloxicam (0.03%) reduces croton oil-induced increases in TNF-α and IL-1β mRNA levels and increases IL-10 mRNA levels in cornea in a rabbit model of acute ocular inflammation. It inhibits pleural plasma exudation in a carrageenan-induced rat model of pleurisy when administered at a dose of 3 mg/kg. In a canine model of unilateral osteoarthritis of the right stifle joint, meloxicam reduces prostaglandin E2 (PGE2) levels in plasma and right stifle joint synovial fluid when administered at a dose of 0.2 mg/kg. Formulations containing meloxicam have been used in the treatment of osteoarthritis and rheumatoid arthritis. | | Clinical Use | In April 2000, the U.S. FDA approved meloxicam for the treatment of osteoarthritis. When meloxicam was initially introduced in the United Kingdom, it was promoted as a selective COX-2 inhibitor. | | Side effects | Meloxicam may cause stomach upset, nausea, dizziness or diarrhoea, increased blood pressure, headache, easy bruising or bleeding; mental or mood changes; signs of kidney problems (e.g., changes in urine output), unexplained stiffness of the neck, symptoms of heart failure (e.g., swelling of the ankles or feet; unusual tiredness, unusual weight gain).
Meloxicam rarely causes serious (potentially fatal) liver disease. Include: nausea or vomiting, loss of appetite, dark-coloured urine, stomach or abdominal pain, and yellowing of the eyes or skin.
Very serious allergic reactions to Meloxicam are rare. Include: fever, swollen lymph nodes, rash, itching or swelling (especially of the face or tongue or throat), severe dizziness, and difficulty breathing. However, if you notice any symptoms of a severe allergic reaction, seek medical help immediately. | | Synthesis | It can be synthesized in four steps from benzisothiazolo-3(2H)-one-1,1-dioxide. Reaction of benzothiazolo-3(2H)-one-1,1-dioxide with methyl chloroacetate gives the methyl 2(3H)-acetate derivative, which is isomerized with sodium methoxide in toluene-tert-butanol yielding methyl 4-hydroxy-2H-1,2-benzothiazine-3-carboxylate-1,1-dioxide. Subsequent methylation with methyl iodide in methanol yields the 2-methyl compound. Finally this compound is treated with 2-amino-5-methylthiazole in xylene. | | Veterinary Drugs and Treatments | Meloxicam is principally used for the symptomatic treatment of osteoarthritis
in dogs. Short-term (single dose injectable) use is also
approved (in the USA) for cats for the control of postoperative pain
and inflammation associated with orthopedic surgery, ovariohysterectomy
and castration when administered prior to surgery. | | Metabolism | Meloxicam, however, is less
selective than celecoxib and much less selective than rofecoxib in in vitro studies.
Meloxicam is readily absorbed when administered orally and is highly bound to plasma proteins. Meloxicam is
extensively metabolized in the liver, primarily by CYP2C9 and, to a lesser extent, by CYP3A4. The advantages of
meloxicam over celecoxib and rofecoxib in the treatment of osteoarthritis (or rheumatoid arthritis) are not readily
apparent. | | Mode of action | Meloxicam, a nonsteroidal anti-inflammatory drug (NSAID), by shutting down prostaglandin synthesis, it has antiinflammatory, antipyretic and analgesic properties. This is accomplished by preferentially inhibiting the COX-2 system relative to the COX-I which also leads to an improved GI safety profile relative to naproxen, diclofenac and prioxicam. It can also interfere with neutrophil function like degranulation. Meloxicam did not inhibit proteoglycan synthesis in osteroarthitic cartilage or chondrocytes and had no effect on platelet aggregation. It is metabolized by the P450 2C9 system into four metabolites which are all inactive. | | References | [1] https://en.wikipedia.org/wiki/Meloxicam
[2] https://www.drugbank.ca/drugs/DB00814
[3] https://www.drugs.com/meloxicam.html
[4] http://www.medicinenet.com/meloxicam/article.htm
[5] K OGINO. Evaluation of pharmacological profile of meloxicam as an anti-inflammatory agent, with particular reference to its relative selectivity for cyclooxygenase-2 over cyclooxygenase-1.[J]. Pharmacology, 1997, 55 1: 44-53. DOI:10.1159/000139511.
[6] CHRISTOPHER J JONES. In vivo effects of meloxicam and aspirin on blood, gastric mucosal, and synovial fluid prostanoid synthesis in dogs.[J]. American journal of veterinary research, 2002, 63 11: 1527-1531. DOI:10.2460/ajvr.2002.63.1527.
[7] S NOBLE J A B. Meloxicam.[J]. Drugs, 1996, 51 3: 424-430; discussion 431-32. DOI:10.2165/00003495-199651030-00007.
[8] BANDOO C CHATALE Mariam S D. Synthesis and in-vivo taste assessment of meloxicam pivalate.[J]. Drug Development and Industrial Pharmacy, 2019, 45 10: 1590-1598. DOI:10.1080/03639045.2019.1628250. |
| | Meloxicam Preparation Products And Raw materials |
| Raw materials | thiazine-->2-Amino-5-methylthiazole-->D-Glucitol, 1-deoxy-1-(methylamino)-, compd. with 4-hydroxy-2-methyl-N-(5-methyl-2-thiazolyl)-2H-1,2-benzothiazine-3-carboxamide 1,1-dioxide (1:1)-->2H-1,2-Benzothiazine-3-carboxamide, 4-hydroxy-2-methyl-N-(5-methyl-2-thiazolyl)-, 1,1-dioxide, potassium salt, hydrate (1:1:1)-->methyl 3-oxo1,2-benzisothiazole-2(3H)-acetate 1,1-dioxide-->Despyridyl Piroxicam (Piroxicam Impurity C)-->Methyl 4-hydroxy-2H-1,2-benzothiazine-3-carboxylate 1,1-dioxide-->Ethyl 4-hydroxy-2-methyl-2H-1,2-benzothiazine-3-carboxylate 1,1-dioxide |
|