- Cefotaxime sodium
-

- $0.00 / 5kg
-
2024-04-23
- CAS:64485-93-4
- Min. Order: 5kg
- Purity: ≥85%
- Supply Ability: 5tons
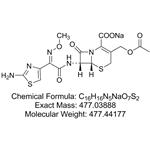
- Cefotaxime Sodium
-
- $0.00 / 10mg
-
2024-04-10
- CAS:64485-93-4
- Min. Order: 10mg
- Purity: 90%+
- Supply Ability: 10g
- Cefotaxime sodium
-

- $30.00/ kg
-
2024-03-31
- CAS:64485-93-4
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 2000000
|
| | Cefotaxime sodium Chemical Properties |
| Melting point | 162-163 C | | alpha | D20 +55±2° (c = 0.8 in water) | | refractive index | 61 ° (C=1, H2O) | | storage temp. | Keep in dark place,Inert atmosphere,2-8°C | | solubility | H2O: aqueous solutions of pH 4.3-6.2 are stable up to 3 weeks at 2-8 °C.soluble | | form | powder | | color | white to yellow | | PH | pH(100g/l, 25℃) : 4.5~6.5 | | Water Solubility | Soluble in water. | | Merck | 14,1933 | | BRN | 5711411 | | Stability: | Stable. Incompatible with strong oxidizing agents. |
| | Cefotaxime sodium Usage And Synthesis |
| Brand Name(s) in US | Claforan
| | Description | Cefotaxime was synthesized by Hoechst and Roussel-Uclaf in 1977. It was the first derivative of cephalosporin to introduce the methoxyimino and aminothiazole groups at the 7 position of the cephem nucleus. Although it shows unexpectedly low oral absorption, its excellent activity against a wide range of gram-positive and gram-negative organisms, including Serratia, Enterobacter, Citrobacter, and anaerobes, guided research and development of the newer synthetic cephems, the so-called thirdgeneration cephalosporins. | | Chemical Properties | White to pale yellow crystalline powder | | Originator | Claforan,Hoechst-Roussel,W. Germany,1980 | | Uses | Cefotaxime sodium salt acts as a beta-lactamase resistant antibiotic. It is used as an effective antibacterial against gram-negative bacteria, with the notable exception of pseudomonas and penicillin-resistant strains of streptococcus pneumoniae. It is used to treat infections of the bones, joints, skin, respiratory tract and blood stream. | | Uses | Cephalosporin antibacterial | | Uses | Broad spectrum third generation cephalosporin antibiotic. The name Cefotaxime applies to the isomer having a syn-methoxy imino group | | Definition | ChEBI: A cephalosporin organic sodium salt having acetoxymethyl and [2-(2-amino-1,3-thiazol-4-yl)-2-(methoxyimino)acetyl]amino side-groups. | | Manufacturing Process | A solution of 8 g of sodium bicarbonate in about 20 ml of ethanol was
progressively added to 45.55 g of pure 3-acetoxymethyl-7-[2-(2-amino-4-
thiazolyl)-2-methoxyiminoacetamido]-ceph-3-eme-4-carboxylic acid in 100 ml
of distilled water and another 80 ml of ethanol and 4.5 g of activated carbon
were added thereto. The mixture was stirred for 5 minutes and was filtered.
The filter was rinsed with ethanol and the filtrate was evaporated to dryness
under reduced pressure. The residue was taken up in 100 ml of ethanol and
evaporated to dryness again. The residue was dissolved in 100 ml of methanol
and the solution was poured into 2 l of acetone. The mixture was vigorously
stirred and was vacuum filtered. The recovered product was rinsed with
acetone and then ether and dried under reduced pressure to obtain 43.7 g of
a white product which rehydrated in air to obtain a final weight of 45.2 g of
sodium 3-acetoxymethyl-7-[2-(2-amino-4-thiazolyl)-2-
methoxyiminoacetamido]-ceph-3-eme-4-carboxylate. | | Brand name | Claforan
(Sanofi Aventis). | | Therapeutic Function | Antibiotic | | General Description | Pharmaceutical secondary standards for application in quality control provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards | | Biological Activity | mic: <0.1 μg/ml for s. pneumoniaecefotaxime is a cephalosporin antibiotic.the cephalosporins, a class of β-lactam antibiotics originally derived from the fungus acremonium, are indicated for the prophylaxis and treatment of infections caused by bacteria susceptible to this particular form of antibiotic. | | Biochem/physiol Actions | Cefotaxim inhibits bacterial cell wall synthesis by binding to penicillin-binding proteins (PBPs) which inhibits the final transpeptidation step of peptidoglycan synthesis in bacterial cell walls. As a result, bacteria lyse due to cell wall autolytic enzymes. | | Clinical Use | Cefotaxime (Claforan) was the first third-generationcephalosporin to be introduced. It possesses excellentbroad-spectrum activity against Gram-positive and Gramnegativeaerobic and anaerobic bacteria. It is more activethan moxalactam against Gram-positive organisms. Manyβ-lactamase–producing bacterial strains are sensitive to cefotaxime,including N. gonorrhoeae, Klebsiella spp., H. influenzae,S. aureus, and E. cloacae. Some, but not all,Pseudomonas strains are sensitive. Enterococci and Listeriamonocytogenes are resistant.
The syn-isomer of cefotaxime is significantly more activethan the anti-isomer against β-lactamase–producing bacteria.This potency difference is, in part, because of greaterresistance of the syn-isomer to the action of β-lactamases.The higher affinity of the syn-isomer for PBPs, however,may also be a factor.
Cefotaxime is metabolized in part to the less active desacetylmetabolite. Approximately 20% of the metaboliteand 25% of the parent drug are excreted in the urine. Theparent drug reaches the cerebrospinal fluid in sufficient concentrationto be effective in the treatment of meningitis.Solutions of cefotaxime sodium should be used within 24hours. If stored, they should be refrigerated. Refrigerated solutionsmaintain potency up to 10 days. | | Veterinary Drugs and Treatments | In the United States, there are no cefotaxime products approved
for veterinary species but it has been used clinically in several
species when an injectable 3rd generation cephalosporin may be
indicated. | | in vitro | previous studies found that the in-vitro activity of cefotaxime against staphylococcus au reus has ranged from 0.8 g to 8 μg/ml with 50% of isolates inhibited by 2 μg/ml and 90% by 4 μg/ml. moreover, it was found that methicillin resistant s. aureus were resistant to cefotaxime with mic values above 64 μg/ml. the cefotaxime mics against s. pneumoniae were found to be below 0.1 μg/ml, with 90% inhibited by 0.04 μg/ml. cefotaxime has also been shown to have excellent activity against haemophilus injluenzae, such as β-lactamase-containing strains [1]. | | in vivo | an in-vivo study with the mouse model of vibrio vulnificus infection was conducted to evaluate the efficacies of therapy with minocycline or cefotaxime alone and in combination. results indicated that combination therapy with cefotaxime and minocycline was distinctly more advantageous than therapy with the single antibiotic regimen for the treatment of severe vibrio vulnificus infections [2]. | | references | [1] neu hc. the in vitro activity, human pharmacology, and clinical effectiveness of new beta-lactam antibiotics. annu rev pharmacol toxicol. 1982;22:599-642.
[2] chuang yc, ko wc, wang st, liu jw, kuo cf, wu jj, huang ky. minocycline and cefotaxime in the treatment of experimental murine vibrio vulnificus infection. antimicrob agents chemother. 1998 jun;42(6):1319-22.
[3] schleupner cj, engle jc. clinical evaluation of cefotaxime for therapy of lower respiratory tract infections. antimicrob agents chemother. 1982 feb;21(2):327-33. |
| | Cefotaxime sodium Preparation Products And Raw materials |
|