|
|
| | TETRAETHYLAMMONIUM PERCHLORATE Basic information |
| Product Name: | TETRAETHYLAMMONIUM PERCHLORATE | | Synonyms: | TETRAETHYLAMMONIUM PERCHLORATE;n,n,n-triethyl-ethanaminiuperchlorate;tetraethylammoniumperchlorate,[dry];TETRAETHYLAMMONIUM PERCHLORATE,ELECTRO- CHEMICAL GRADE;Tetraethylammonium perchlorate, 98% (Assay, on a dry basis), wet with ca 10% water;Tetraethylammonium perchlorate, 98% (Assay, on a dry basis), wet with 10% water;TETRAETHYLAMMONIUM PERCHLORATE, 0.2M AQUEOUS SOLUTION;N,N,N-Triethylethanaminium·perchlorate | | CAS: | 2567-83-1 | | MF: | C8H20ClNO4 | | MW: | 229.7 | | EINECS: | 219-904-3 | | Product Categories: | Supporting Electrolytes for Electrochemistry;Ammonium SaltsSynthetic Reagents;Greener Alternatives: Catalysis;Oxidation;Perchlorates;Phase Transfer Catalysts;Ammonium SaltsAnalytical Reagents;Electrochemistry | | Mol File: | 2567-83-1.mol | 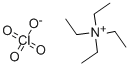 |
| | TETRAETHYLAMMONIUM PERCHLORATE Chemical Properties |
| Melting point | >300°C | | form | Wet crystals | | Water Solubility | Soluble in water, acetone, acetonitrile and dimethyl sulfoxide. | | BRN | 3638093 | | Stability: | Unstable. Heating may lead to an explosion. Contact with combustible material may cause fire. Incompatible with reducing agents, organic materials, finely powdered metals, aluminium, sulfur, benzene, calcium hydride, charcoal, alkenes, ethanol, strontium hydride, sulfuric acid. | | CAS DataBase Reference | 2567-83-1(CAS DataBase Reference) | | EPA Substance Registry System | Tetraethylammonium perchlorate (2567-83-1) |
| | TETRAETHYLAMMONIUM PERCHLORATE Usage And Synthesis |
| Chemical Properties | solid | | Uses | Tetraethylammonium perchlorate is used as supporting electrolyte in polarographic measurements. | | General Description | White crystalline solid. May explode from heat, shock, or friction. | | Air & Water Reactions | Water soluble. | | Reactivity Profile | Oxidizing agents, such as TETRAETHYLAMMONIUM PERCHLORATE, can react with reducing agents to generate heat and products that may be gaseous (causing pressurization of closed containers). The products may themselves be capable of further reactions (such as combustion in the air). The chemical reduction of materials in this group can be rapid or even explosive, but often requires initiation (heat, spark, catalyst, addition of a solvent). Explosive mixtures of inorganic oxidizing agents with reducing agents often persist unchanged for long periods if initiation is prevented. Such systems are typically mixtures of solids, but may involve any combination of physical states. Some inorganic oxidizing agents are salts of metals that are soluble in water; dissolution dilutes but does not nullify the oxidizing power of such materials. Organic compounds, in general, have some reducing power and can in principle react with compounds in this class. Actual reactivity varies greatly with the identity of the organic compound. Inorganic oxidizing agents can react violently with active metals, cyanides, esters, and thiocyanates. Benzene, calcium hydride, charcoal, ethanol, finely divided metals, olefins, strontium hydride, sulfur react violently with perchlorates. | | Purification Methods | Crystallise the perchlorate repeatedly from water, aqueous MeOH, acetonitrile or acetone, and dry it at 70o under a vacuum for 24hours. [Cox et al. J Am Chem Soc 106 5965 1984, Liu et al. J Am Chem Soc 108 1740 1986, White & Murray J Am Chem Soc 109 2576 1987.] It has also been crystallised twice from ethyl acetate/95% EtOH (2:1) [Lexa et al. J Am Chem Soc 109 6464 1987]. [Beilstein 4 IV 332.] |
| | TETRAETHYLAMMONIUM PERCHLORATE Preparation Products And Raw materials |
|