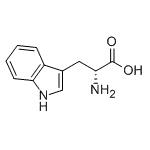
- D(+)-Tryptophan
-

- $50.00 / 1KG
-
2024-04-12
- CAS:153-94-6
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 10000 Mt/Year
- D(+)-Tryptophan
-
- $0.00 / 1kg
-
2024-04-07
- CAS:153-94-6
- Min. Order: 0.10000000kg
- Purity: 99
- Supply Ability: 20tons
- D-tryptophan
-

- $30.00/ KG
-
2023-10-08
- CAS:153-94-6
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 20T
|
| | D(+)-Tryptophan Chemical Properties |
| Melting point | 282-285 °C (dec.)(lit.) | | Boiling point | 342.72°C (rough estimate) | | alpha | 31.5 º (c=1, H2O 24 ºC) | | density | 1.1754 (rough estimate) | | refractive index | 31 ° (C=1, H2O) | | storage temp. | Keep in dark place,Inert atmosphere,Room temperature | | solubility | Aqueous Base (Slightly), DMSO (Slightly, Heated, Sonicated), Methanol (Slightly) | | pka | 2.30±0.10(Predicted) | | form | Powder | | color | White to slightly yellow | | Water Solubility | 11 g/L (20 ºC) | | BRN | 86198 | | Stability: | Stable. Incompatible with oxidizing agents. | | InChIKey | QIVBCDIJIAJPQS-SECBINFHSA-N | | LogP | 0.704 (est) | | CAS DataBase Reference | 153-94-6(CAS DataBase Reference) | | EPA Substance Registry System | D-Tryptophan (153-94-6) |
| | D(+)-Tryptophan Usage And Synthesis |
| Description | D(+)-tryptophan is the D-form (non-proteinogenic form) of the amino acid tryptophan. It can be used for Human embryonic kidney cell (HEK-293, ATCC: CRL-1573) culture. It is also a sweetener used to study the release of incretins from enteroendocrine cells triggered by sugar but not by sweeteners.
| | Description | D-tryptophan (Full name D (+)-Tryptophan) is a white to pale yellow or white crystalline powder at room temperature, odorless or slight odor, slightly sweet taste. Solubility in water is 1.14 g (25 ℃), soluble in dilute acid and alkali, stable in alkali, decomposed in strong acid, slightly soluble in ethanol, not soluble in chloroform, ethyl ether. It plays an important role for human and animal growth and development, metabolism, known as the second essential amino acids. They are almost identical with L-type and physical and chemical properties, and only the optical activity is opposite. But their distribution, function and coenzyme have diversity. Its melting point is very high, generally above 200 degrees. It can dissolve in water, and in the near UV region it has the ability to absorb light.
Tryptophan is one of the 20 standard amino acids, as well as an essential amino acid in the human diet. It is encoded in the standard genetic code as the codon UGG. the D-stereoisomer is occasionally found in naturally produced peptides (for example, the marine venom peptide contryphan). The distinguishing structural characteristic of tryptophan is that it contains an indole functional group. It is an essential amino acid as defined by its growth effects on rats. | | Characteristics and Functions | D-tryptophan amino acid, as a non-reactive protein, has special physiological properties. It can be used as a non-nutritive sweeteners, feed additive, plant growth agents in food feed industry and agriculture. In the pharmaceutical industry, it mainly used for the synthesis of various polypeptides, instead of L-tryptophan peptide drug for half-life extending and reduce the side effects, but it will not resistant to the front body, and it will become important enzyme inhibitor. It can also improve the body's immunity, delayed allergic reactions. Most peptide antibiotic can resistant gram-positive bacteria, Gram-negative bacteria and some, such as Pseudomonas aeruginosa, mycobacteria, fungi, bacteria, pathogens and tumor cells, which have better inhibit and kill ability.
D-tryptophan can be used for the synthetic of semi-synthetic antibiotics, for which pharmacological plays an important role for the side chain. The peptide bond is difficult to be β-lactam enzyme action, thus high stability, and has broad antibacterial spectrum, toxicity, hypoallergenic, rapid absorption, high blood concentrations of the drug long duration. | | Project Standards | Appearance white or slightly yellow crystalline powder
Content 99.0%~101.0%
Specific rotation [α] D20 + 30.5 °~+ 32.5 °
Transmittance ≥95.0%
Acidity PH 5.5~6.4
Loss on drying ≤0.2%
Residue on ignition ≤0.1%
Chloride [Cl-] ≤0.02%
Sulphate [SO42-] ≤0.02%
Ammonium [NH4 +] ≤0.02%
Heavy metals [Pb] ≤10ppm
Ferric [Fe] ≤10ppm
Arsenic salt [As2O3] ≤1ppm
Other amino acids compliance | | Application | An important nutrient, niacin is used as the control agent Medicine
Production method: D-tryptophan as raw material, chloroacetic anhydride was added under cooling state after polishing sulfuric acid in ice water, filtered and recrystallized from water, and finally treatment with carboxypeptidase trypsin to remove L-type, acidified with acetic acid, ethanol recrystallization refining. | | References | https://pubchem.ncbi.nlm.nih.gov/compound/D-Tryptophan#section=Top | | References | Fujita, Yukihiro, et al. "Incretin release from gut is acutely enhanced by sugar but not by sweeteners in vivo." American Journal of Physiology Endocrinology & Metabolism 296.3(2009):E473.
Bacchus, W, W. Weber, and M. Fussenegger. "Increasing the dynamic control space of mammalian transcription devices by combinatorial assembly of homologous regulatory elements from different bacterial species." Metabolic Engineering 15.1(2013):144.
| | Chemical Properties | white powder | | Uses | D-Tryptophan is used for human embryonic kidney cell (HEK-293, ATCC: CRL-1573) culture. The product is a sweetener used to study the release of incretins from enteroendocrine cells triggered by sugar but not by sweeteners. | | Uses | An essential amino acid found in naturally produced pedtides. Unlike its stereoisomer, L-tryptophan, it is not used in structural or enzyme proteins. | | Definition | ChEBI: The D-enantiomer of tryptophan. | | General Description | White solid. | | Air & Water Reactions | Slightly soluble in water. | | Reactivity Profile | Amines are chemical bases. They neutralize acids to form salts plus water. These acid-base reactions are exothermic. The amount of heat that is evolved per mole of amine in a neutralization is largely independent of the strength of the amine as a base. Amines may be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen is generated by amines in combination with strong reducing agents, such as hydrides. | | Fire Hazard | Flash point data are not available for D(+)-Tryptophan, but D(+)-Tryptophan is probably combustible. | | Biochem/physiol Actions | Low level of D-tryptophan is capable of preventing the growth of an?L-tryptophan-requiring mutant of the?E. coli. |
| | D(+)-Tryptophan Preparation Products And Raw materials |
|