- Furosemide
-
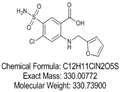
- $0.00 / 10mg
-
2024-04-10
- CAS:54-31-9
- Min. Order: 10mg
- Purity: 90%+
- Supply Ability: 10g
- Furosemide
-

- $10.00 / 1kg
-
2024-03-22
- CAS:54-31-9
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 20000tons
- Furosemide
-

- $35.00/ kg
-
2024-03-16
- CAS:54-31-9
- Min. Order: 1kg
- Purity: 99.8%
- Supply Ability: 200tons/year
Related articles - Side effects of Furosemide
- Furosemide is the diuretic of choice in acute pulmonary oedema or other states of fluid overload (e.g. cardiac, renal or liver....
- Feb 24,2022
|
| | Furosemide Chemical Properties |
| Melting point | 220 °C (dec.) (lit.) | | Boiling point | 582.1±60.0 °C(Predicted) | | density | 1.606 | | refractive index | 1.6580 (estimate) | | Fp | 11 °C | | storage temp. | 2-8°C | | solubility | Practically insoluble in water, soluble in acetone, sparingly soluble in ethanol (96 per cent), practically insoluble in methylene chloride. It dissolves in dilute solutions of alkali hydroxides. | | pka | pKa 3.8 (Uncertain) | | form | powder | | color | White to Off-White | | Water Solubility | Soluble in acetone, DMF or methanol. Slightly soluble in water | | Merck | 14,4309 | | BCS Class | 2 (CLogP), 4
(LogP) | | Stability: | Stable, but light sensitive, air sensitive and hygroscopic. Incompatible with strong oxidizing agents. | | InChIKey | ZZUFCTLCJUWOSV-UHFFFAOYSA-N | | CAS DataBase Reference | 54-31-9(CAS DataBase Reference) | | IARC | 3 (Vol. 50) 1990 | | EPA Substance Registry System | Furosemide (54-31-9) |
| | Furosemide Usage And Synthesis |
| Efficient diuretics | Furosemide, is a class of efficient sulfonamide diuretics acting on the medullary loop of the ascending branch of the medulla,it has a strong and short-term diuretic effect,which can increase the excretion of water, sodium, chloride, potassium, calcium, magnesium, phosphate and so on. It Mainly inhibits Na + and Cl-reabsorption in medullary and cortex of the medullary loop ascending branch crude segment , it can promote the excretion of sodium, chloride and potassium and affect the formation of renal medullary high osmotic pressure,it can interfer the process of concentration and dilution of urine, and it can increase urine output. This product can inhibit the activity of prostaglandin decomposition enzyme ,make the content of prostaglandin E2 increase,it has effect on expansion of blood vessels, it also plays a role in the proximal tubule, glomerular filtration,it can increase renal blood flow,and adjust renal blood flow distribution,and reduce blood flow so that the cortex surface blood flow increases,it promotes diuresis, its effect is fast and strong, it is used for other diuretics invalid cases. Clinically it is used for the treatment of cardiac edema, renal edema, cirrhosis ascitic fluid, peripheral edema caused by dysfunction or vascular disorders , and it may contribute to an upper urinary tract stones excretion. Administration intravenously can treat brain edema, it also can accelerate the excretion of toxic substances in cerebral edema in poisoning . Note that when the diuretic furosemide is used, since the excretion of urine Cl-, Na +, K +, H + is increasing, while the excretion of HCO3-is not increasing, long-term repeated drug use or large quantities of drugs can cause low salt syndrome, low chlorine and low potassium alkalosis.
In recent years, researchers find that inhalation of furosemide can have a significant effect on asthma, it is like cromolyn sodium, which through inhibition of allergic mediator release, it can inhibit the release of the neurotransmitter acetylcholine and substance P, which may be related to inhibiting chloride ions into the cell membranes of respiratory tract. Clinically,it is used in exercise-induced asthma, immediate and delayed type antigen-induced asthma.
The above information is edited by the Chemicalbook of Tian Ye.
| | Chemical properties | white or white alike crystalline powder. 206 ℃ melting point. Dissolved in acetone, methanol, dimethyl formamide, slightly soluble in ethanol, insoluble in water. Odorless, almost tasteless.
| | Uses | The diuretic effect of this product is a strong and short,it is a potent diuretic for the treatment of edema caused by heart, liver, kidney and other diseases, in particular, the base cases which other diuretics are invalid to;it can be used to treat acute pulmonary edema, brain edema , acute renal failure and high blood pressure and other diseases; in combination with fluid infusion, the product can promote poison excretion. Rat oral LD50 is 2600-2820mg/kg.
| | production method | 2,4-dichlorobenzoic acid (see 12740) by sulfochlorination,ammoniation, acidification ,dichloro-5-sulfamoyl-benzoic acid is obtained. Then after condensation with the chaff amine , furosemide is produced.
| | Category | Toxic substances
| | Toxicity grading | Middle toxic
| | Acute toxicity | Oral-rat LD50: 2600 mg/kg; Oral-Mouse LD50: 2200 mg/kg.
| | Flammability and hazard characteristics | Combustible; fire decomposition produces toxic nitrogen oxides; sulfur oxides and chlorides smoke.
| | Storage Characteristics | Ventilated, low-temperature ,dry storeroom.
| | Extinguishing agent | Water, carbon dioxide, dry powder,sandy soil.
| | Description | Furosemide (Item No. 26298) is an analytical reference standard categorized as a diuretic. Formulations containing diuretics, including furosemide, have been misused in sports for weight reduction and as masking agents in humans and to prevent exercise-induced pulmonary hemorrhage in racehorses. This product is intended for use in analytical forensic applications. This product is also available as a general research tool . | | Chemical Properties | white to light yellow crystal powde | | Originator | Lasix,Hoechst,W. Germany,1964 | | Uses | An inhibitor of NKCC and a GABAA receptor antagonist. | | Uses | This compound belongs to the aminobenzenesulfonamides. These are organic compounds containing a benzenesulfonamide moiety with an amine group attached to the benzene ring. | | Uses | diuretic, antihypertensive | | Uses | Furosemide inhibits ion co-transport in the kidney. Furosemide is used as a diuretic. | | Definition | A benzoic-sulfonamide-furan. It is a diuretic with
fast onset and short duration and anti-hypertensive
agent. | | Manufacturing Process | 10.8 grams of 3-sulfamyl-4,6-dichlorobenzoic acid (0.04 mol) and 11.7 grams
of furfurylamine (0.12 mol) are heated in 30 cc of diethyleneglycol�dimethylether for 6 hours under reflux. When pouring the reaction mixture
into 300 cc of 1 N hydrochloric acid, the reaction product is immediately
separated off in the form of crystals. The light-yellow crude product is purified
by dissolving it in 100 cc of warm 1 N sodium bicarbonate solution,
precipitation by means of hydrochloric acid and subsequent recrystallization
from ethanol/water, with addition of charcoal. Colorless prisms are obtained
which decompose at 206°C while adopting a brown coloration, and with
evolution of gas. | | Brand name | Lasix (Sanofi Aventis). | | Therapeutic Function | Diuretic | | General Description | Odorless white to slightly yellow crystalline powder. A diuretic drug. Almost tasteless. | | Air & Water Reactions | Light sensitive. Air sensitive. Slightly soluble in water. | | Reactivity Profile | Furosemide may undergo hydrolysis at sufficiently low pH. The pH of aqueous solutions should be maintained in the basic range to prevent hydrolysis. Alcohol has been shown to improve the stability of Furosemide. Incompatible with strong oxidizing agents . | | Hazard | Poison; moderately toxic; teratogen; questionable carcinogen; mutagen. | | Fire Hazard | Flash point data for Furosemide are not available; however, Furosemide is probably combustible. | | Biological Activity | Loop diuretic that inhibits the Na + /2Cl - /K + (NKCC) cotransporter. Also acts as a non-competitive antagonist at GABA A receptors with ~ 100-fold greater selectivity for α 6-containing receptors than α 1-containing receptors. | | Biochem/physiol Actions | Inhibits ion co-transport in the kidney. | | Mechanism of action | Furosemide is a highly effective and quick-acting diuretic whose action, like all of the
examined loop diuretics, is associated with blocking reabsorption of ions in the ascending
bend of Henle’s loop. It is used for edema syndrome of various origins, edema of the lungs
and brain, chronic renal insufficiency, some forms of hypertonic crises, and poisoning by
barbiturates and other compounds excreted mainly with urine. | | Clinical Use | Furosemide has a saluretic effect 8- to 10-fold that of the thiazide diuretics; however, it has a shorter duration of action (~6–8 hours). Furosemide causes a marked
excretion of sodium, chloride, potassium, calcium, magnesium, and bicarbonate ions, with as much as 25% of the filtered load of sodium excreted in response to initial
treatment. It is effective for the treatment of edemas connected with cardiac, hepatic, and renal sites. Because it lowers the blood pressure similar to the thiazide
derivatives, one of its uses is in the treatment of hypertension. | | Side effects | Clinical toxicity of furosemide and other loop diuretics primarily involves abnormalities of fluid and electrolyte balance. As with the thiazide diuretics, hypokalemia is an
important adverse effect that can be prevented or treated with potassium supplements or coadministration of potassium-sparing diuretics. Increased calcium ion excretion
can be a problem for postmenopausal osteopenic women, and furosemide generally should not be used in these individuals. Hyperuricemia, glucose intolerance,
increased serum lipid levels, ototoxicity, and gastrointestinal side effects might be observed as well. Hypersensitivity reactions also are possible with furosemide (a
sulfonamide-based drug), and cross-reactivity with other sulfonamide containing drugs is possible. | | Safety Profile | Poison by intravenous
route. Moderately toxic by ingestion and
intraperitoneal routes. Human systemic
effects by intravenous route: change in the
sensitivity of the ear to sound, tinnitus,
unspecified effects on the heart, constriction
of the arteries, a decrease in urine volume,
interstitial nephritis, metabolic alkalosis,
pulse rate decrease, fall in blood pressure.
Ingestion can damage the liver.
Experimental teratogenic and reproductive
effects. Questionable carcinogen with
experimental carcinogenic effects. Human
mutation data reported. When heated to
decomposition it emits very toxic fumes of
Cl-, NOx, and SOx. | | Synthesis | Furosemide, 4-chloro-N-furfuryl-5-sulfamoylanthranylic acid (21.4.11), is
synthesized in a relatively simple manner from 2,4-dichlorobenzoic acid, which is converted
into 5-aminosulfonyl-4,6-dichlorobenzoic acid (21.4.10) during subsequent reaction
with chlorosulfonic acid and ammonia. Reacting this with furfurylamine gives
furosemide (21.4.11) . 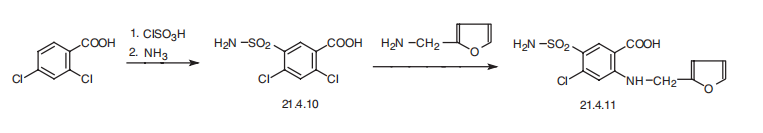
| | Veterinary Drugs and Treatments | Furosemide is used for its diuretic activity in all species. It is used
in small animals
for the treatment of congestive cardiomyopathy,
pulmonary edema, hypercalcuric nephropathy, uremia, as adjunctive
therapy in hyperkalemia and, occasionally, as an antihypertensive
agent. In cattle,
it is approved for use for the treatment of
post-parturient udder edema. It has been used to help prevent or
reduce epistaxis (exercise-induced pulmonary hemorrhage; EIPH)
in racehorses. | | Drug interactions | Potentially hazardous interactions with other drugs
Analgesics: increased risk of nephrotoxicity with
NSAIDs; antagonism of diuretic effect with
NSAIDs.
Anti-arrhythmics: risk of cardiac toxicity with
anti-arrhythmics if hypokalaemia occurs; effects of
lidocaine and mexiletine antagonised.
Antibacterials: increased risk of ototoxicity with
aminoglycosides, polymyxins and vancomycin; avoid
with lymecycline.
Antidepressants: increased risk of hypokalaemia with
reboxetine; enhanced hypotensive effect with MAOIs;
increased risk of postural hypotension with tricyclics.
Antiepileptics: increased risk of hyponatraemia with
carbamazepine; effects antagonised by phenytoin.
Antifungals: increased risk of hypokalaemia with
amphotericin.
Antihypertensives: enhanced hypotensive effect;
increased risk of first dose hypotensive effect
with alpha-blockers; increased risk of ventricular
arrhythmias with sotalol if hypokalaemia occurs.
Antipsychotics: increased risk of ventricular
arrhythmias with amisulpride or pimozide (avoid
with pimozide) if hypokalaemia occurs; enhanced
hypotensive effect with phenothiazines.
Atomoxetine: hypokalaemia increases risk of
ventricular arrhythmias.
Cardiac glycosides: increased toxicity if hypokalaemia
occurs.
Ciclosporin: variable reports of increased
nephrotoxicity, ototoxicity and hepatotoxicity.
Cytotoxics: concentration of furosemide increased
by dasabuvir, ombitasvir and paritaprevir - reduce
furosemide dose; increased risk of ventricular
arrhythmias due to hypokalaemia with arsenic
trioxide; increased risk of nephrotoxicity and
ototoxicity with platinum compounds.
Lithium: risk of toxicity. | | Metabolism | Little biotransformation of furosemide takes place. It
is mainly eliminated via the kidneys (80-90%); a small
fraction of the dose undergoes biliary elimination and
10-15% of the activity can be recovered from the faeces. | | storage | Store at RT | | references | [1]. hochman dw. the extracellular space and epileptic activity in the adult brain: explaining the antiepileptic effects of furosemide and bumetanide. epilepsia, 2012, 53 suppl 1: 18-25.
[2]. chen h, sun d. the role of na-k-cl co-transporter in cerebral ischemia. neurol res, 2005, 27(3): 280-286.
[3]. prandota j. furosemide: progress in understanding its diuretic, anti-inflammatory, and bronchodilating mechanism of action, and use in the treatment of respiratory tract diseases. am j ther, 2002, 9(4): 317-328. |
| | Furosemide Preparation Products And Raw materials |
|