|
|
| Product Name: | 2,2'-Azobis(2-methylpropionitrile) | | Synonyms: | A,A'-AZOISOBUTYRONITRILE;ALPHA,ALPHA'-AZOBISISOBUTYRONITRILE;ALPHA,ALPHA'-AZO-DI-ISO-BUTYRONITRILE;ALPHA,ALPHA'-AZOISOBUTYRONITRILE;2,2'-dicyano-2,2'-azopropane;2,2'-Dimethyl-2,2'-azodipropionitrile;2,2’-azobis[2-methylpropanenitrile];2,2-Azobsisobutyronitril | | CAS: | 78-67-1 | | MF: | C8H12N4 | | MW: | 164.21 | | EINECS: | 201-132-3 | | Product Categories: | Free Radical Initiators;Organics;pharmacetical;78-67-1 | | Mol File: | 78-67-1.mol | 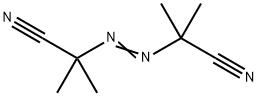 |
| | 2,2'-Azobis(2-methylpropionitrile) Chemical Properties |
| Melting point | 102-104 °C (dec.)(lit.) | | Boiling point | 281.68°C (rough estimate) | | density | 1.11 | | vapor pressure | 0.81Pa at 24.85℃ | | refractive index | n20/D1.495 | | Fp | 4℃ | | storage temp. | 2-8°C | | solubility | Chloroform (Slightly), Methanol (Slightly) | | form | Solid | | color | Crystals from EtOH | | Odor | odorless | | Water Solubility | Insoluble | | Merck | 13,920 | | BRN | 1708400 | | Stability: | Stability Flammable solid. Shock sensitive. Thermally unstable. May be explosive in combination with acetone or heptane. Incompatible with oxidizing agents. | | InChIKey | OZAIFHULBGXAKX-VAWYXSNFSA-N | | LogP | 1.1 at 25℃ | | CAS DataBase Reference | 78-67-1(CAS DataBase Reference) | | NIST Chemistry Reference | 2,2'-Azo-bis-isobutyronitrile(78-67-1) | | EPA Substance Registry System | Azobis(isobutyronitrile) (78-67-1) |
| | 2,2'-Azobis(2-methylpropionitrile) Usage And Synthesis |
| Description | Azobisisobutyronitrile (abbreviated AIBN) is an organic compound with the formula [(CH3)2C(CN)]2N2. This white powder is soluble in alcohols and common organic solvents but is insoluble in water. It is often used as a foamer in plastics and rubber and as a radical initiator. It's soluble in a wide variety of organic solvents, including alcohol-based solvents. Insoluble in water and denser than water. Moderately toxic by ingestion. Readily ignited by sparks or flames. Burns intensely and persistently. Toxic oxides of nitrogen produced during combustion. Used as a catalyst, in vinyl polymerizations and a blowing agent for plastics.
| | Uses | 2,2'-Azobis(2-methylpropionitrile) is used as an initiator in polymer radical polymerization because its molecules can easily undergo split reactions and form molecules with high activation energy. 2,2'-Azobis(2-methylpropionitrile) (AIBN) is the most commonly used azo initiator. Its characteristic is that the decomposition reaction is relatively stable, only one kind of free radical is generated, and basically no induced decomposition occurs, so it is often used in the kinetics research of free radical polymerization.
| | Uses | Azobisisobutyronitrile (AIBN) is an azo-compound and is widely used as a free radical initiator. This compound has labile carbon-nitrogen covalent bond which undergoes homolytic scission under thermal, chemical or photochemical conditions producing free radicals. They are useful in many reactions like halogenation, polymerisation of vinyl monomers, grafting reactions, curing of rubbers and unsaturated polymers and cross-linking of polyolefins. AIBN can be used as an initiator in the synthesis of highly cross-linked Poly(divinylbenzene) (PDVB) polymers. It also can be used as an initiator in the polymerization process of 2-hydroxyethyl methacrylate (HEMA).
| | Recrystallization | 1, recrystallize twice a chemically pure 2,2'-Azobis(2-methylpropionitrile) with methanol (by 1:12) and air-dry in a ventilated dark overnight, then place in a vacuum oven with phosphorus pentoxide as a desiccant, vacuum degree 1.013 * 105Pa, under reduced pressure, and dry to 24h.Refined 2,2'-Azobis(2-methylpropionitrile) needs to be put in the colored jar, sealed and kept in cold dark place.
2, add 50ml 95% alcohol into 150ml Erlenmeyer flask equipped with a reflux condenser , heat in a water bath to near boiling, quickly add 5gAIBN, shak to make it be completely dissolved (boiling time not too long, if too long, severe decomposition ), filter hot solution rapidly ( the funnel and filter flask using for filter must be warmed) and cool the filtrate to give white crystals, dry in a vacuum desiccator, a melting point of 102℃, store the product in a brown bottle, cryopreservation.
| | Temperature of initiator | 2,2'-Azobis(2-methylpropionitrile) is a particularly excellent free radical initiator , nitrogen will be released by the decomposition when it is heated to about 70 ° C and free radicals (CH3) 2CCN is generated which is affected by cyano radical , more stable. It can react with other organic substrates, and then generates a new radical in annihilation of itself , causing chain reaction of free radicals (see radical reaction). At the same time, it can also be two coupled molecules, generating highly toxic tetramethoxysilane succinonitrile (TMSN).
AIBN melts when heated to 100~107 ° C and dramaticly decomposes, releases nitrogen and several organic nitrile compound toxic to humans, and may cause an explosion, fire. Slowly decomposes at room temperature, and it should be stored at 10 ° C or less. Away from fire, heat source. poisonous. Metabolizes into hydrocyanic acid in the blood, liver, brain and other tissues of animals.
The above information is edited by the Chemicalbook of Tian Ye.
| | Chemical properties | White columnar crystals or white crystalline powder. Insoluble in water, soluble in methanol, ethanol, acetone, ether, petroleum ether and aniline and other organic solvents.
| | Uses | The polymerization initiator for Vinyl chloride, vinyl acetate, acrylonitrile and blowing agent for rubber, plastic , in an amount of 10% to 20%.
This product can also be used as curing agents, pesticides and organic synthesis intermediates.
This product is a highly toxic substance, mice are orally LD5017.2~25mg/kg, the organic cyanide released by the decomposition when it is heated has a greater poison on the human body .
| | Production method | Acetone, hydrazine hydrate and sodium cyanide as raw materials:
The temperature of condensation reaction above is 55~60 ℃, reaction time is 5h, and then cool down to 25~30 ℃ ,time is 2h. When to be cooled to below 10 ℃,begin to flow chlorine and carry out the reaction at below 20 ℃.
Ratio of material: HCN: acetone: hydrazine = 1L:1.5036kg:0.415kg.
Acetone cyanide alcohol and hydrazine hydrate react, and then the use of chlorine oxidation or amino nitrile with sodium hypochlorite oxidation.
| | category | Flammable solids
| | Toxicity grading | Middle toxic
| | Acute toxicity | Oral-rat LDL0: 670 mg/kg; Oral-Mouse LD50: 700 mg/kg | | Explosive hazardous characteristics | Explosive when mixed with oxidants ; easily oxidized, unstable and intense heat decomposition, explosive when heated with heptane, acetone
| | Flammability hazard characteristics | Flammable in case of fire, high temperature, oxidant ; decomposition and generating combustible gas in case of thermal ; combustion produces toxic fumes of nitrogen oxides
| | Storage characteristics | Treasury ventilation low-temperature drying; and separated from oxidants
| | Extinguishing agent | Water, dry sand, carbon dioxide, foam, 1211 fire extinguishing agent
| | Description | Azobisisobutylonitrile is a white crystallinecompound. Molecular weight= 164.21; Specific gravity(H2O:1)= 1.11 at 25℃; Boiling point= decomposes;Freezing/Melting point= 99°103℃ (decomposes);Autoignition temperature= 63℃. NFPA 704 M HazardIdentification (based on NFPA-704M Rating System):Health 3, Flammability 2, Reactivity 2. Insoluble in water. | | Chemical Properties | Azobisisobutylonitrile is a white crystalline
compound. | | Uses | foaming agent and inhibitor in plastic and elastomer materials | | Uses | Used as an initiator in the synthesis of highly cross-linked Poly(divinylbenzene) (PDVB) polymers. 1 Used as an initiator in the polymerization process of 2-hydroxyethyl methacrylate (HEMA). 2 | | Uses | 2,2′-Azobis(2-methylpropionitrile) (AIBN) is a radical initiator. AIBN solution can be used to initiate radical-induced reactions, specifically free-radical polymerizations. It can be used in:
- Synthesis of styrene-vinyl pyridine diblock copolymers by reversible addition-fragmentation chain transfer (RAFT) polymerization.
- Preparation of silicon oxycarbide glasses.
- Synthesis of poly [N-(p-vinyl benzyl) phthalimide] for the preparation of titanium dioxide composites for electrophoretic displays.
| | Definition | ChEBI: (E)-Azobis(isobutyronitrile) is an azo compound. | | General Description | Insoluble in water and denser than water. Moderately toxic by ingestion. Readily ignited by sparks or flames. Burns intensely and persistently. Toxic oxides of nitrogen produced during combustion. Used as a catalyst, in vinyl polymerizations and a blowing agent for plastics. | | Air & Water Reactions | Dust may form an explosive mixture in air. Insoluble in water. | | Reactivity Profile | Self-decomposition or self-ignition may be triggered by heat, chemical reaction, friction or impact. Self-accelerating decomposition may occur if the specific control temperature is not maintained. These materials are particularly sensitive to temperature rises. 2,2'-Azobis(2-methylpropionitrile) is an azo compound. Azo, diazo, azido compounds can detonate. This applies in particular to organic azides that have been sensitized by the addition of metal salts or strong acids. Toxic gases are formed by mixing materials of this class with acids, aldehydes, amides, carbamates, cyanides, inorganic fluorides, halogenated organics, isocyanates, ketones, metals, nitrides, peroxides, phenols, epoxides, acyl halides, and strong oxidizing or reducing agents. Flammable gases are formed by mixing materials in this group with alkali metals. Explosive combination can occur with strong oxidizing agents, metal salts, peroxides, and sulfides. | | Hazard | Toxic by ingestion. | | Flammability and Explosibility | Not classified | | Safety Profile | Poison by
intraperitoneal route. Moderately toxic by
ingestion. Easily oxidized, unstable. Violent
exothermic decomposition when heated.
Solution in acetone may decompose
explosively. Explodes when heated with
heptane. When heated to decomposition it
emits toxic fumes of NO, and CN-. See also
NITRILES. A free-radcal generator. | | Potential Exposure | Azobisisobutylonitrile is both a nitrile
and azo compound. Used as a polymerization initiator, free
radical generator (or initiator); as a catalyst in vinyl polymerizations;
as a blowing agent for elastomers and plastics. | | First aid | If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit.Note: Use amyl nitrate capsules if symptoms develop. Allarea employees should be trained regularly in emergencymeasures for cyanide poisoning and in CPR. A cyanide antidote kit should be kept in the immediate work area and mustbe rapidly available. Kit ingredients should be replaced every1°2 years to ensure freshness. Persons trained in the use ofthis kit, oxygen use, and CPR must be quickly available. | | storage | Color Code—Red Stripe: Flammability Hazard: Donot store in the same area as other flammable materials. Priorto working with this chemical you should be trained on itsproper handling and storage. Azodiisobutyronitrile must bestored to avoid contact with acetone, lithium, aluminumhydride, and water, since violent reactions occur. Azodiisobutyronitrile is self-reactive and will explode at elevated temperatures. It should be stored under nitrogen, dry ice, or ice.Sources of ignition, such as smoking and open flames, areprohibited where this chemical is used, handled, or stored in amanner that could create a potential fire or explosion hazard. | | Shipping | UN3234 Self-reactive solid type C, temperature
controlled materials, Hazard Class: 4.1; Labels: 4.1-
Flammable solid, Technical Name Required. | | Incompatibilities | Flammable; dust may form explosive
mixture with air. Unstable and easily oxidized material;
keep away from oxidizers, strong acids. Keep at temperature
not ≧30° C (this may vary by manufacturer). Risk of
explosion from heat, shock, friction. Warming causes production
of tetramethylsuccinonitrile and cyanide fumes.
Keep away from acetone and other ketones, alcohols, lithium,
aluminum, aldehydes, and hydrocarbons, such as heptane.
Azo compounds can detonate. This applies in
particular to organic azides that have been sensitized by the
addition of metal salts or strong acids. Toxic gases are
formed by mixing materials of this class with acids, aldehydes,
amides, carbamates, cyanides, inorganic fluorides,
halogenated organics, isocyanates, ketones, metals, nitrides,
peroxides, phenols, epoxides, acyl halides, and strong oxidizing
or reducing agents. Flammable gases are formed by
mixing materials in this group with alkali metals. Explosive
combination can occur with strong oxidizing agents, metal
salts, peroxides, and sulfides. This chemical is sensitive to
prolonged exposure to heat. This chemical is incompatible
with strong oxidizing agents. |
| | 2,2'-Azobis(2-methylpropionitrile) Preparation Products And Raw materials |
| Raw materials | Acetone-->Chlorine-->Hydrazinium hydroxide solution-->Sodium cyanide-->Sodium hypochlorite-->Acetone cyanohydrin-->BUTYRONITRILE | | Preparation Products | 4-(PYRROLIDIN-1-YLMETHYL)BENZENEBORONIC ACID, PINACOL ESTER 97%1-[4-(4,4,5,5-TETRAMETHYL-1,3,2-DIOXABOROLAN-2-YL)BENZYL]PYRROLIDINE-->4-(2-CYANOETHYLAMINOCARBONYL)PHENYLBORONIC ACID-->2,1,3-Benzothiadiazole-5-carbaldehyde-->3-(2-CYANOETHYLAMINOCARBONYL)PHENYLBORONIC ACID-->4-(PIPERIDINE-1-CARBONYL)PHENYLBORONIC ACID-->2,1,3-Benzothiadiazol-5-ylmethanol-->3-(PIPERIDINOMETHYL)BENZALDEHYDE-->4-(1,2,3-THIADIAZOL-4-YL)BENZYLAMINE HYDROCHLORIDE-->3-PHENOXYPHENYLACETONITRILE-->2,6-Dichlorobenzaldehyde-->5-Dibenzosuberenone-->2-Chlorobenzyl chloride-->4-(1,2,3-THIADIAZOL-4-YL)BENZALDEHYDE-->3-QUINOLIN-2-YLPROPANOIC ACID-->Dichloroacetyl chloride-->Dicofol-->4-Benzofurazancarboxaldehyde-->2-(DIPHENYLPHOSPHINO)ETHYLTRIETHOXYSILANE-->5-(BROMOMETHYL)-2,1,3-BENZOTHIADIAZOLE-->Deflazacort-->Dideoxyinosine-->graft copolymer PAA-G-PIPA microsphere-->2-(Bromomethyl)benzoic acid-->2-TRIFLUOROMETHYLQUINOLINE-7-CARBOXYLIC ACID-->4-[4-(BROMOMETHYL)PHENYL]-1,2,3-THIADIAZOLE-->3-CHLORO-4-((CYCLOPROPYLAMINO)METHYL)BENZALDEHYDE-->METHYL ALPHA-BROMO-2-CHLOROPHENYLACETATE-->Maleic acid-allyl alcohol copolymer-->11-Bromoundecanoic acid-->1-(3-CHLORO-4-((CYCLOPROPYLAMINO)METHYL)PHENYL)ETHANONE-->2,4-Dichlorobenzyl chloride-->super absorbent resin synthesized by microwave cation ion radiation-->3,7-dichloro-8-dichloro methyl quinoline-->METHYL 4-(BROMOMETHYL)-3-METHOXYBENZOATE-->dimethylacrolyl phenoxy propanestyrene copolymer optical plastics-->2-Nitroacetophenone-->amphoteric super absorbent resin synthesized by microwave radiation-->FTHALIDE |
|