- Doxazosin mesylate
-

- $0.00 / 25Kg/Bag
-
2024-04-10
- CAS:77883-43-3
- Min. Order: 2Kg/Bag
- Purity: USP
- Supply Ability: 20 tons
- Doxazosin mesylate
-

- $0.00 / 1KG
-
2024-03-16
- CAS:77883-43-3
- Min. Order: 100g
- Purity: 98%+
- Supply Ability: 100kg
- Doxazosin mesylate
-

- $2857.00 / 1KG
-
2023-06-15
- CAS:77883-43-3
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 100kg
|
| | Doxazosin mesylate Chemical Properties |
| Melting point | 275-277°C | | storage temp. | 2-8°C | | solubility | Slightly soluble in water, soluble in a mixture of 15 volumes of water and 35 volumes of tetrahydrofuran, slightly soluble in methanol, practically insoluble in acetone. It shows polymorphism (5.9), some forms may be hygroscopic | | form | powder | | color | white | | BCS Class | 1 | | Stability: | Protect from light | | InChIKey | VJECBOKJABCYMF-UHFFFAOYSA-N | | CAS DataBase Reference | 77883-43-3(CAS DataBase Reference) |
| | Doxazosin mesylate Usage And Synthesis |
| Description | Doxazosin mesylate is a quinazoline compound that is a selective inhibitor of the alpha1 subtype of alpha adrenergic receptors. The chemical name of doxazosin mesylate is 1-(4-amino-6,7-dimethoxy-2-quinazolinyl)-4-(1,4-benzodioxan-2-ylcarbonyl) piperazine methanesulfonate.The empirical formula for doxazosin mesylate is C23H25N505 • CH4O3S and the molecular weight is 547.6.
Doxazosin mesylate is freely soluble in dimethylsulfoxide, soluble in dimethylformamide, slightly soluble in methanol, ethanol, and water (0.8% at 25°C), and very slightly soluble in acetone and methylene chloride. Each doxazosin mesylate tablet, for oral administration, contains 1 mg, 2 mg, 4 mg and 8 mg of doxazosin as the free base.
Doxazosin mesylate is a new generation of quinazolone α1 receptor blocker developed by the Pfizer company (United States), It has a long half-life, exerting its effects of dilating the blood vessels, reducing the vascular resistance and lowering the blood pressure through blocking the a 1 receptor. It selectively blocks the a1 adrenergic receptor of the prostate smooth muscle matrix, capsule and bladder neck, alleviating the symptoms of patients of benign prostatic hyperplasia, while having good curing effect on the dysuria caused by simple prostatic hyperplasia. In 1988, doxazosin, as a drug for the treatment of hypertension, had been listed in Denmark. In 1995, the US FDA had approved it for the treatment of benign prostatic hyperplasia. In 2002 September, Doxazosin controlled release tablets had been listed in China, providing a new option for the domestic treatment of benign prostatic hyperplasia. It has been recommended abroad as first-line clinical drugs of anti-hypertension and treatment of prostate disease. | | Pharmacological effects | This product is a new highly selective α1 receptor blockers, having a significant effect of causing reduction in blood pressure while having good effect of alleviating lipid metabolism. It can significantly reduce serum triglycerides and total cholesterol; it can also selectively block the a1 adrenergic receptor of prostate smooth muscle matrix, capsule and bladder neck, alleviating the symptoms of benign prostatic hyperplasia patients, further significantly slowing down the effect of benign prostatic hyperplasia, especially having good effect on the treatment of dysuria caused by simple prostatic hyperplasia. It has a long half-life. | | Synthetic route | 1. Reaction bottle was added of catechol and sodium hydroxide solution, stir evenly and dropped of epichlorohydrin at 50 ℃. The dripping process can be finished within 0.5 h. After the completion of dripping, the solution was refluxed at 85 ℃ for 2.5 h, after which the solution turned from dark green to brown oil. It is further extracted with chloroform, and the organic phases are combined, followed by being dried over anhydrous sodium sulfate, filtered, and the filtrate is concentrated under reduced pressure, and the residue is cooled to crystallize, filtered. The filter cake is further washed with a small amount of cold carbon tetrachloride and dried at below 50 ℃ to obtain a white solid compound 2.
2. Potassium permanganate and potassium hydroxide solution were added to the reaction flask, and the pyridine solution of Intermediate 2 was added dropwise at 0-10 °C. After the completion of the dropping, the reaction was continued for 24 h at room temperature. The reaction mixture was filtered. The filtrate has its pH adjusted by concentrated ammonia to 7, concentrated to half, added of chloroform after cooling. The concentrated hydrochloric acid was added dropwise of concentrated hydrogen chloride and adjusted to pH = 1. It is then extracted with chloroform. The organic phases were combined, dried over anhydrous sodium sulfate, filtered and the filtrate was concentrated under reduced pressure to give Compound 3 as a pale yellow solid.
3. The reaction flask was added with intermediate 3, toluene solution and DMF. The mixture was heated to 50 ° C, stirred and dissolved, and thionyl chloride was added dropwise. The reaction mixture was refluxed for 4 hours. The toluene and the remaining thionyl chloride were distilled off under reduced pressure to give the crude compound 4.
4. Add piperazine, methanol and water to the reaction flask, stir the mixture evenly, add the ethyl acetate solution of Intermediate 4 dropwise at 10-20 ℃, continue the reaction for 2 hours, extract the reaction mixture with methylene chloride. The organic phase was extracted twice with water and the aqueous phase was adjusted to pH 10 with concentrated aqueous ammonia. The organic phase was extracted three times with dichloromethane. The organic phases were combined, washed twice with water, dried over anhydrous sodium sulfate, filtered and evaporated of methylene chloride under reduced pressure to give the compound 5 as a pale yellow solid.
5. The reaction flask was added with 2-chloro-4-amino-6, 7-dimethoxyquinazoline, intermediate 5 and n-butanol, stirred and refluxed for 4 hours. After completion of the reaction, the crystals were cooled and washed with n-butanol, and dried to obtain Compound 6 as a white crystalline powder.
6 is placed into the beaker, adjusted to pH = 10 with ammonia; extract it with dichloromethane; remove the water layer; wash the organic phase with water; slowly add methanesulfonic acid and gradually precipitate of white crystals, apply cooling crystallization, filtration and drying to obtain the crude product 1.
7. The crude product is added to the methanol, heated to dissolve all; cool and crystallize it; filter, wash with cold methanol once, dry to obtain the refined product 1.
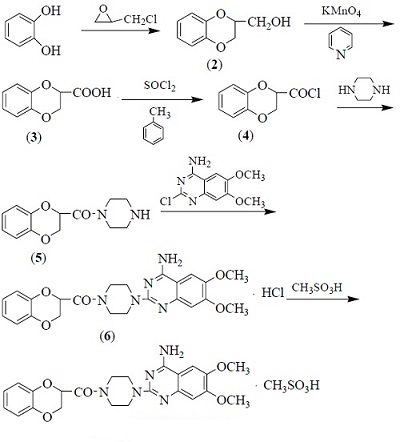
Figure 1 shows the synthesis route of doxazosin mesylate | | Usage and dosage |
- Commonly adult oral dose, apply an initial dose of 1mg, once a day. After 1-2 weeks, adjust the dosage according to the clinical response and tolerance; for first dose and dose adjustment, it is preferably taken at bedtime. Maintenance dose of 1-8mg, once a day, but over 4 mg can cause orthostatic hypotension. Foreign research data suggest that the maximum dose of this product to 16mg/day.
- Pediatric dose has not been determined.
| | Pharmacokinetics | The product should be administrated orally with oral bioavailability of 62% to 69%, protein binding rate of 98% to 99%, peak time of 1.7 to 3.6 hours, elimination half-life of 16 to 22 hours. It should subject to liver metabolism. About 65% of the drug was eliminated by metabolites via fecal, and only about 5% of the prototype was excreted by urine. | | Side effects | There may be dizziness, dry mouth, headache, palpitations, fatigue, the symptoms are mild, bearable, disappear within about 1 week, without special treatment. No postural hypotension occurred. | | Precautions | Patients allergic to this product should be disabled. During the postmarketing experience of treatment of hypertension, there are reports of tachycardia, palpitations, chest pain, angina pectoris, myocardial infarction, cerebrovascular accident and arrhythmia, but in general, those symptoms can’t be distinguished from those of patients don’t take doxazosin. Application of this product may affect the capability of driver and the mechanical operator, especially during the initial drug phase. | | Application | Patients of primary mild to moderate hypertension, and hypertensive patients with benign prostatic hypertrophy; For patients who have difficulty controlling blood pressure with a single drug, doxazosin can be administered in combination with thiazide diuretics and beta-blockers, calcium antagonists, or angiotensin converting enzyme inhibitors. It can be used for treatment of high blood pressure.
The outline, synthetic route, usage, dosage, pharmacokinetics and adverse reaction of doxazosin mesylate were compiled by Baoquan of Chemicalbook. (2016-04-09) | | References | https://dailymed.nlm.nih.gov/dailymed/archives/fdaDrugInfo.cfm?archiveid=51901
https://www.drugs.com/doxazosin.html | | Chemical Properties | White or almost white crystalline powder. | | Originator | Alfadil,Roerig/Pfizer,Sweden | | Uses | A selective a-1-adrenergicblocker related to prazosin | | Uses | Doxazosin ER is indicated for the treatment of the signs and symptoms of benign prostatic hyperplasia | | Uses | A selective α-1-adrenergicblocker related to prazosin. | | Uses | antihypertensive | | Uses | Doxazosin is a selective α1-adrenoceptor antagonist. Doxazosin relaxes smooth muscles of the prostate. | | Manufacturing Process | 4-Amino-2-chloro-6,7-dimethoxyquinazoline (140 g) and N-(1,4-benzodioxan-
2-carbonyl)piperazine (150 g) were stirred together under reflux in n-butanol
(2 L) for 3.5 hours. The mixture was then cooled to 80°C, the solid product
collected, washed with cold n-butanol (2 times 250 ml), and dried. The crude
product was dissolved in hot (80°C) dimethylformamide (530 ml) and water
(130 ml), filtered, concentrated in vacuo to about 300 ml, then cooled and
ether (1.8 L) added. The solid so obtained was collected and washed with
ether to give 4-amino-2-[4-(1,4-benzodioxan-2-carbonyl)piperazin-1-yl]-6,7-
dimethoxyquinazoline hydrochloride (215 g), melting point 289°C-290°C.
Mesylate may be prepared by usual method from hydrochloride with
methylsulphonic acid. | | Brand name | Cardura (Pfizer). | | Therapeutic Function | Adrenergic blocker | | Biological Activity | Selective α 1 -adrenoceptor antagonist (pK i values are 9.0, 8.5 and 8.4 for human α 1B , α 1A and α 1D receptors respectively). Displays antihypertensive activity. | | Biochem/physiol Actions | α1-adrenoceptor antagonist; relaxes smooth muscles of the prostate | | storage | Desiccate at RT | | references | [1] zhao y1, cao xb, ren lm. doxazosin selectively potentiates contraction to serotonin via 5-ht₂a receptors in longitudinal muscle strips of the rabbit gastric body. can j physiol pharmacol. 2014 mar;92(3):197-204.
[2] o'neil ml1, beckwith le, kincaid cl, rasmussen dd. the α1-adrenergic receptor antagonist, doxazosin, reduces alcohol drinking in alcohol-preferring (p) rats. alcohol clin exp res. 2013 feb;37(2):202-12.
[3] tung d1, ciallella j, cheung ph, saha s. novel anti-inflammatory effects of doxazosin in rodent models of inflammation. pharmacology. 2013;91(1-2):29-34. |
| | Doxazosin mesylate Preparation Products And Raw materials |
|