- Sodium sulfide
-

- $0.00 / 1TON
-
2023-12-25
- CAS:1313-82-2
- Min. Order: 1TON
- Purity: 60%
- Supply Ability: 1000000
- Sodium sulfide
-
- $50.00 / 1KG
-
2023-12-23
- CAS:1313-82-2
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: g-kg-tons, free sample is available
- Sodium sulfide
-

- $1.00 / 25KG
-
2023-12-12
- CAS:1313-82-2
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 2000tons per month
Related articles - Sodium sulfide: Uses and Safety
- Sodium sulphide is commonly used in the pulp and paper industry for the manufacture of paper products. It is used as a bleachi....
- Dec 28,2023
- The uses of sodium sulfide
- Sodium sulfide is the chemical compound with the formula Na?S, or more commonly its hydrate Na?S·9H?O. Both are colorless wate....
- Mar 4,2020
|
| | Sodium sulfide Chemical Properties |
| Melting point | 950 °C(lit.) | | density | 1.86 g/mL at 25 °C(lit.) | | storage temp. | Refrigerator (+4°C) | | solubility | H2O: 0.1 g/mL, clear, colorless | | form | flakes | | Specific Gravity | 1.856 | | color | Yellow | | Water Solubility | 186 g/L (20 ºC) | | Sensitive | Hygroscopic | | Merck | 14,8681 | | Dielectric constant | 5.0(Ambient) | | Stability: | Spontaneously flammable. Incompatible with acids, metals, oxidizing agents. Contact with acid liberates toxic gas. Fine dust/air mixtures are explosive. Hygroscopic. | | InChIKey | CXPWOVUZRAFMDA-UHFFFAOYSA-N | | CAS DataBase Reference | 1313-82-2(CAS DataBase Reference) | | NIST Chemistry Reference | Sodium sulfide(1313-82-2) | | EPA Substance Registry System | Sodium sulfide (1313-82-2) |
| | Sodium sulfide Usage And Synthesis |
| Chemical Properties | Sodium sulfide is the chemical compound with the formula Na₂S, or more commonly its hydrate Na₂S·9H₂O. Both are colorless water-soluble salts that give strongly alkaline solutions. When exposed to moist air, Na₂S and its hydrates emit hydrogen sulfide, which smells like rotten eggs. Sodium sulfide, anhydrous is a yellow to brick red crystalline mass or fused solid. If exposed to moist air, it absorbs moisture from the air, and it is liable to spontaneous heating and may cause ignition of nearby combustible material. The industrial sodium sulfide often exhibits pink, reddish brown or yellowish brown color for containing impurities.
| | Sodium sulfide |
Sodium sulfide(1313-82-2) is also known as smelly soda and stinky base. At room temperature, the pure product is colorless or slightly purple prismatic crystal. The industrial sodium sulfide often exhibits pink, reddish brown or yellowish brown color for containing impurities. It has rotten egg smell and is corrosive and toxic. Its density is 2.427. It will be subject to decomposition at 920 ℃. It is soluble in cool water and easily soluble in hot water with dissolving in water almost fully being hydrolyzed into sodium hydroxide and sodium hydrosulfide (at 10 ℃, the solubility is 15.4; while the solubility is 57.2 g at 90 ℃). The aqueous water exhibits strongly alkalinity and is corrosive on copper, wood, skin, etc. It is slightly soluble in ethanol but insoluble in ether. When being encountered strong acid, the sodium sulfide will release hydrogen sulfide. It will be subject to deliquescence in air and is easily oxidized into sodium thiosulfate. Sodium sulfide is mainly used as the raw materials of hides depilatories, pulp cooking agent, and sulfur dye, the reducing agents of dye intermediates, fabric dyeing mordant, and ore flotation agent. It can also be used as viscose fiber desulfurizer and the raw material for production of sodium hydrosulfide and sodium polysulfide.
The sodium sulfide in our county was originated in the 1830s. The production of it was earliest started from a chemical plant in Dalian, Liaoning in small-scale. Upon entering into the mid-1980s to the 1990s, with the vigorous development of the international chemical industry, the domestic industry had undergone a fundamental transformation with both the number and scale of production being dramatically increased with rapid development. The production area of sodium sulfide centered in Shanxi Yuncheng has quickly expanded to a dozen of other provinces or cities including Yunnan, Xinjiang, Inner Mongolia, Gansu, Qinghai, Ningxia, and Shaanxi. The national annual production capacity has increased from the value of 420,000 tons by the end of the 80s to a value of 640,000 tons in mid-1990s. The region with the fastest growing includes Inner Mongolia, Gansu, and Xinjiang region at northwest of China. The production capacity has reached 200,000 tons in Inner Mongolia, which has become the largest production base of sodium sulfide in China.

Figure 1 is a picture of yellow flaky sodium sulfide
The above information is edited by the Chemicalbook of Dai Xiongfeng.
| | Industrial sodium sulfide |
Industrial sodium sulfide(1313-82-2) is generally a mixture with different numbers of crystalline water; the molecular formula is Na2S ? nH2O; it exhibits as yellow or reddish-brown massive, flaky and granular and is mainly used in paper, dyes, mineral processing, printing and dyeing industries.
GB/T 10500-2000 standard has classified the industrial sodium sulfide products into three categories: Category 1 is ordinary sodium sulfide (commonly known as red base); Class 2 is low-iron sodium sulfide (commonly known as the yellow base); Class 3 is sodium sulfide of high content.
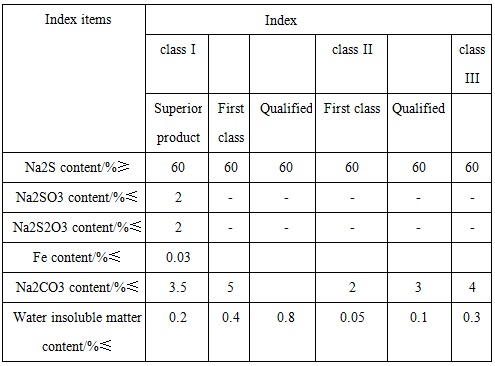
Figure 2 the reference quality indicators of industrial sodium sulfide
Packing, storage and shipping: sodium sulfide belongs to alkaline corrosive substances, classification: GB 8.2 class, number:82011. It is packed with a tight leakproof iron drums with the net weight per barrel being 25.50 kg or 100 kg. The packaging should contain obvious "drugs" and "corrosive substance" signs. It should be stored in a dry, airy shed asbestos with the container must be intact. It can’t be stored and shipped together with acidic materials and oxidizing agents.
| | Toxicity | Sodium sulfide is strongly corrosive to the skin. Worker subjecting to contact with a solution of sodium sulfide has their hand skin get ruffling and redness. During the operation, you should note that: upon inadvertently contact with skin, you should rinse with water. After the sodium sulfide droplets or small pieces falling into eyes, immediately wash with water for 15 min and send to hospital for treatment. To protect the skin, it is recommended to wash hands with a weak acetic acid solution and then coated with oily ointment. Pay attention to the protection of eye.
| | Preparation of polyarylene sulfide | We can take industrial sodium sulfide and poly-halogenated aromatic compounds as raw materials; apply multi-component composite catalyst or additive and carry out segmented poly-condensation at normal pressure in high-boiling polar organic solvent (such as hempa) for generating linear high molecular weight polyarylene sulfide. The reaction conversion rate is high with the product being white granular and with excellent mechanical properties, thermal properties and thermal processing stability. Additionally supplement of a certain amount of cross-linking agent can generate higher molecular weight branched or cross-linked polyarylene sulfide.
| | Chemical Properties | It is pale yellow flakes with the main features being the iron (Fe2O3) content is lower than 30 mg/kg. See other properties in sodium sulfide.
| | Uses | It can be used for the production of high-grade sulfur dye and high-quality leather. It can be used in the production of high-grade paper production in the paper industry.
In the dye industry, it can be used for the production of sulfur dye and is the raw material of sulfur green and sulfur blue. In the printing and dyeing industry, it can be used as a facilitator agent for dissolving sulfur dye. In the tanning industry, it can be used for the hydrolysis for hair removal of raw skin and also used for the preparation of sodium polysulfide to help accelerate soaking and softening of dry skin. It can also be used as the cooking agent of papermaking industry. In fiber textile industry, it is used for the denitrofication of artificial fiber textile as well as the reduction of nitration as well as the mordant for cotton fabric dyeing. In the pharmaceutical industry, it is mainly used for the production of antipyretic drugs such as phenacetin. In addition, it can also be used for making sodium thiosulfate, sodium hydrosulfide, and sodium polysulfide.
It can be used for analysis reagents and leather depilatory
It can be used for the manufacture of dyes, sulfide, and used as ore flotation agent, depilatory rawhide, and the cooking agent of paper.
| | Production method | Pulverized coal reduction method: put mirabilite and coal powder in a mixing ratio of 100: (21 to 22.5) (weight ratio) for calcination and reduction at 800~1100 ℃. The resultant after cooling is molten into a liquid. After standing for clarification, the upper portion of the alkaline solution was concentrated to obtain a solid sulfide. The flake (or granules)-like sodium sulfide is made through the transition tank and flaking. The reaction equation is as below:
Na2SO4 + 2C → Na2S + 2CO2
Absorption method: use 380~420 g/L sodium hydroxide solution to absorb H2S> 85% containing hydrogen sulfide waste gas; the resulting product was concentrated by evaporation to obtain sodium sulfide products. Its reaction equation is as below:
H2S + 2NaOH → Na2S + 2H2O
Barium sulfide method: use sodium sulfide and barium sulfide for cross-metathesis reaction to get precipitated barium sulfate. During this process, we can get the byproduct sodium sulfide. The reaction formula is as below:
BaS + Na2SO4 → Na2S + BaSO4 ↓
Gas reduction method: in the presence of iron catalyst, put hydrogen (or carbon monoxide, coal gas, methane gas) into boiling furnace for reaction with sodium sulfate, we can obtain high-quality anhydrous granular sodium sulfide (containing Na2S: 95%~97% ). Its reaction equation is:
Na2SO4 + 4CO → Na2S + 4CO2
Na2SO4 + 4H2 → Na2S + 4H2O
Refined method using the byproduct (4% of sodium sulfide) during the production of precipitated barium sulfate as raw material, pump it into dual-effect evaporator for concentration into 23%; it further enters into the mixing tank for removing iron as well as carbon; pump it into the evaporator (manufactured by pure nickel material) to evaporate the lye into a certain concentration and further send it into the roller squeezing apparatus for flaking and further obtain the finished product after screening and packaging.
Pulverized coal reduction method is the traditional production method for making sodium sulfide. During the manufacturing process, improve the equipment and materials and increase the iron removal process so that the products can meet standards.
| | Category | corrosive materials
| | Toxicity grading | highly toxic
| | Acute toxicity | oral-rat LD50: 208 mg/kg; Oral-Mouse LD50: 205 mg/kg
| | Hazardous characteristics of explosive | it is explosive upon heating and collision.
| | Flammability and hazard characteristics | it release toxic hydrogen sulfide gas upon acids; anhydrous sodium sulfide is flammable; heating can release toxic fumes of sulfur oxides
| | Storage characteristics | Treasury: ventilation, low-temperature and dry; store it separately from oxidants and acids
| | Extinguishing agent | water, sand
| | Chemical Properties | Sodium sulfide,Na2S, also known as sodium sulfuret,is an irritating, water-soluble, yellowish to reddish, deliquescent powder that melts at 1180°C (2156 °F). Sodium sulfide is used as a chemical intermediate and solvent,in conversion of wood into paper pulp, as a photographic and analytical reagent,as a source of sulfide,as a reducing agent,in organic reactions, as a depilatory, and in sheep dips. | | Physical properties | White cubic crystal; hygroscopic; density 1.856 g/cm3; melts at 1,172°C; soluble in water 18.6 g/100mL at 20°C and 39 g/100mL at 50°C; aqueous solutions strongly alkaline; slightly soluble in alcohol; insoluble in ether.
The pentahydrate consists of flat, shiny prismatic crystals; density 1.58 g/cm3; loses three water molecules at 100°C; melts at 120°C losing all water molecules; soluble in water and alcohol; aqueous solutions strongly alkaline; insoluble in ether.
The nonahydrate is a yellowish-white crystalline solid; tetragonal crystals; odor of hydrogen sulfide; the color changes on exposure to light and air, first turning to yellow and then becoming brownish-black, deliquescent; density 1.43 g/cm3; decomposes at about 50°C; very soluble in water; aqueous solution strongly alkaline; slightly soluble in alcohol; insoluble in ether. | | Uses | These yellow flakes were made by fusing sodium carbonate with
sulfur. Soluble in water but less so in alcohol, sodium sulfide
was lovingly called “stink” by those who used it for toning
prints or intensifying negatives because of its sulfurous smell. | | Uses | It is used for H2S therapy, to study its effect on the prevention of diabetes in animals. | | Uses | Sodium sulfide (Na2S) is used in the dye industry, in the oxidation process of gold, lead, and
cooper metal ores, as a sheep dip, and to process paper. | | Uses | used in sulfur dyes, used as reductant, mordant, floatation agent, depilatory for leather, digestion auxiliary in paper-making, and used in textile, pigment and rubber | | Definition | A yellow-red solid, Na2S, formed by the reduction of sodium sulphate with carbon (coke) at elevated temperatures. It is a corrosive and readily oxidized material of variable composition and usually contains polysulphides of the type Na2S2, Na2S3, and Na2S4, which cause the variety of colours. It is known in an anhydrous form (r.d. 1.85; m.p. 1180°C) and as a nonahydrate, Na2S·9H2O (r.d. 1.43; decomposes at 920°C). Other hydrates of sodium sulphide have been reported. The compound is deliquescent, soluble in water with extensive hydrolysis, and slightly soluble in alcohol. It is used in wood pulping, dyestuffs manufacture, and metallurgy on account of its reducing properties. It has also been used for the production of sodium thiosulphate (for the photographic industry) and as a depilatory agent in leather preparation. It is a strong skin irritant. | | Preparation | Sodium sulfide is prepared by heating sodium bisulfate with sodium chloride and coal above 950°C. The product mixture is extracted with water and the hydrated sulfide is obtained from the solution by crystallization: NaHSO4 + NaCl + 2C → Na2S + 2CO2↑ + HCl↑
Sodium sulfide also is produced from its elements in liquid ammonia: Na + 2S → Na2S. | | Definition | ChEBI: A sulfide salt with formula Na2S. The pentahydrate and (particularly) the nonahydrate are also known. In gel form, sodium sulfide is used to soften toenails to assist in trimming (and so relive pain) of ingrowing toenails. | | General Description | Sodium sulfide is a yellow to brick red crystalline mass or fused solid with an odor of rotten eggs. If exposed to moist air Sodium sulfide is liable to spontaneous heating and may cause ignition of nearby combustible material. Sodium sulfide absorbs moisture from the air. | | Air & Water Reactions | Aqueous solutions of sodium sulfide when exposed to air slowly convert to sodium hydroxide and sodium thiosulfate. The crystalline form upon exposure to air forms hydrogen sulfide and sodium carbonate [Merck 11th ed. 1989]. | | Reactivity Profile | SODIUM SULFIDE is a white to yellow crystalline material, flammable. Can explode on rapid heating or when shocked. Violent reaction with carbon, charcoal, diazonium salts, N,N-dichloromethylamine, strong oxidizers, water. On contact with acids Sodium sulfide liberates highly toxic and flammable hydrogen sulfide gas. When heated to decomposition Sodium sulfide emits toxic fumes of sodium oxide, and oxides of sulfur [Bretherick, 5th ed., 1995, p. 1729]. | | Hazard | Flammable, dangerous fire and explosion
risk. Strong irritant to skin and tissue, liberates toxic
hydrogen sulfide on contact with acids. | | Health Hazard | Caustic action on skin and eyes. If ingested may liberate hydrogen sulfide in stomach. | | Fire Hazard | Special Hazards of Combustion Products: Irritating sulfur dioxide is produced in fire. | | Flammability and Explosibility | Non flammable | | Industrial uses | In non-metallic flotation, sodium sulfide is also used as a depressant and for collector
desorption, in particular, fatty acids from monazite, pyrochlore, zircon and microcline.
As a depressant for quartz, sodium sulfide is an excellent depressant for iron-activated
quartz as well as non-activated quartz. | | Industrial uses | Sodium sulfide (Na2S·9H2O) is a hygroscopic substance with a specific gravity of 1.864
and a melting temperature of 1180 °C. The reagent is soluble in water. The aqueous solution
of sodium sulfide has a highly alkaline reaction resulting from its hydrolysis:
Na2S + H2O ? NaOH + NaHS | | Safety Profile | A poison by ingestion and intraperitoneal routes. Flammable when exposed to heat or flame. Unstable and can explode on rapid heating or percussion. Reacts violently with carbon, diazonium salts, n,n-dichloromethylamine, onitroaniline diazonium salt, water. When heated to decomposition it emits toxic fumes of SOx and Na2O. See also SULFIDES | | Purification Methods | Some purification of the hydrated salt can be achieved by selecting large crystals and removing the surface layer (contaminated with oxidation products) by washing with distilled water. Other metal ions can be removed from Na2S solutions by passage through a column of Dowex ion-exchange A-1 resin, Na+-form. The hydrated salt can be rendered anhydrous by heating it in a stream of H2 or N2 until water is no longer evolved. (The resulting cake should not be heated to fusion because it is readily oxidised.) Recrystallise it from distilled water [Anderson & Azowlay J Chem Soc, Dalton Trans 469 1986]. Note that sodium sulfide hydrolyses in H2O to form NaHS + H2O, and is therefore alkaline. A 0.1N solution in H2O is 86% hydrolysed at room temperature. Its solubility in H2O is 8% at 0o, 12% at 20o and 30% at 50o. The anhydrous salt is obtained by allowing it to stand in a vacuum over conc H2SO4 or P2O5 at 45o to start with, then at 30-35o when the salt contains 4% of water. The last traces of water are removed by heating to 700o in a glass or porcelain tube in a stream of H2 to give pure H2S. [Fehér in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol I pp 358-360 1963.] |
| | Sodium sulfide Preparation Products And Raw materials |
|