- Lincomycin
-

- $21.00 / 1000Grams
-
2024-04-24
- CAS:154-21-2
- Min. Order: 1000Grams
- Purity: 99.99%
- Supply Ability: 100Tons
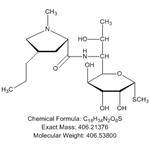
- Lincomycin
-
- $0.00 / 10mg
-
2024-04-10
- CAS:154-21-2
- Min. Order: 10mg
- Purity: 90%+
- Supply Ability: 10g
- Lincomycin
-

- $10.00 / 1kg
-
2023-07-14
- CAS:154-21-2
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 1000tons
|
| | Lincomycin Basic information |
| | Lincomycin Chemical Properties |
| Melting point | 148-150°C | | Boiling point | 646.8±55.0 °C(Predicted) | | density | 1.1704 (rough estimate) | | refractive index | 1.6510 (estimate) | | storage temp. | Hygroscopic, -20°C Freezer, Under inert atmosphere | | solubility | Aqueous Acid (Slightly), DMSO (Slightly), Methanol (Slightly), Water | | form | Solid | | pka | 7.6(at 25℃) | | color | White to Off-White | | Stability: | Hygroscopic | | NIST Chemistry Reference | Lincomycin(154-21-2) | | EPA Substance Registry System | Lincomycin (154-21-2) |
| | Lincomycin Usage And Synthesis |
| Description | Lincomycin was isolated from a strain of Streptomyces lincolnensis in the Upjohn Research Laboratories. Lincosamides are also produced by S. roseolus, S. caelestis, and Monomicrospora halophytica. They consist of an amino acid connected to a sugar by an amide bond. It is available for intravenous, intramuscular, oral, and rectal use. Absorption after oral administration is up to one-third of the dose and plasma protein binding is around 75%. Because of the superior activity and bioavailability of clindamycin, lincomycin is now infrequently used clinically, but it is still available in some countries, in particular for skin and skin structure infections. Thus, the information in this chapter will primarily apply to clindamycin. Many chemical modifications of the lincomycin molecule have been developed and, of these, clindamycin (7-chloro-7-deoxylincomycin) is the most promising and clinically superior to lincomycin. | | Description | Lincomycin is an antibiotic active against grampositive
bacteria. Occupational exposure occurs in
poultry and pig breeders. | | Chemical Properties | White Crystalline Solid | | Originator | Lincocin,Upjohn,UK,1964 | | Uses | An antibiotic produced by Streptomyces lincolnensis. Antibacterial | | Uses | Lincomycin (Clindamycin Phosphate EP Impurity A) is a lincosamide antibiotic that forms cross-links within the peptidyl transferase loop region of the 23S rRNA. Inhibits bacterial protein synthesis. Antibacterial.This compound is a contaminant of emerging concern (CECs). | | Uses | Lincomycin is a polar, water soluble, broad spectrum antibiotic first isolated from Streptomyces licolnensis by researchers at Upjohn in 1962. Lincomycin was the first of a unique structural class, the lincosamides, containing a rare amino acid, 4-propyl-N-methylproline, coupled to an equally rare aminomethylthio-octopyranoside sugar. Lincomycin and semi-synthetic analogues are often incorrectly considered to be aminoglycosides but share little or no structural similarity. Lincomycin is a broad spectrum antibiotic with activity against anaerobic bacteria and protozoans. Lincomycin acts by binding to the 23S ribosomal subunit, blocking protein synthesis. Lincomycin has been extensively studied with over 7,000 literature citations. | | Definition | ChEBI: A carbohydrate-containing antibiotic produced by the actinomyces Streptomyces lincolnensis. | | Manufacturing Process | As described in US Patent 3,086,912, the process comprises cultivating
Streptomyces lincolnensis var. lincolnensis in an aqueous nutrient medium
containing a source of assimilable carbohydrate and assimilable nitrogen
under aerobic conditions until substantial activity is imparted to the medium
by production of lincolnensin and isolating the lincolnensin so produced. | | Brand name | Lincocin (Pharmacia & Upjohn). | | Therapeutic Function | Antibacterial | | Antimicrobial activity | Lincomycin has an antibacterial effect with respect to Gram-positive microorganisms
(staphylococci, streptococci, pneumococci, diphtheria bacillus, and clostridia). It is used
for serious bacterial infections: sepsis, osteomyelitis, septic endocarditis, pneumonia, pul�monary abscess, infected wounds, and purulent meningitis. Lincomycin is a reserve drug
for infections caused by strains of staphylococci and other Gram-positive microorganisms
that are resistant to penicillin and other antibiotics. Synonyms of this drug are lincocin,
mycivin, albiotic, and others. | | General Description | Lincomycin was found in the culture broth of Streptomyces lincolnensis var. lincolnensis by the Upjohn Co. in 1962. It shows antibacterial activity similar to that of the macrolide antibiotics and also shows excellent activity against anaerobic bacteria. Lincomycin is used clinically in combination with other classes of antibiotics for postoperative, gynecological, urinary tract, ear and nose, and other infections. | | Contact allergens | Lincomycin is an antibiotic of the lincosanide group,active against Gram-positive bacteria. Occupational
exposure occurs in poultry and pig breeders | | Clinical Use | Lincomycin is a natural product isolated from fermentations of Streptomyces lincolnensisvar. lincolnensis. It
is active against Gram-positive organisms, including some anaerobes. It is indicated for the treatment of
serious infections caused by sensitive strains of streptococci, pneumococci, and staphylococci. It generally
is reserved for patients who are allergic to penicillin because of the increased risk of pseudomembranous
colitis. It also serves as the starting material for the synthesis of clindamycin (by a SN-2
reaction that inverts the R stereochemistry of the C-7 hydroxyl to a C-7 S-chloride). | | Synthesis | Lincomycin, 6,8-dideoxy-6-trans-(1-methyl-4-propyl-L-2-pyrrolidincar�boxamido)-1-methylthio-D-erythro-α-D-galacto-octopyranoside (32.5.1), is the first
lincosamide that has found use in clinical practice, and which was isolated in 1962
from the culture liquid of the activity of the actinomycete Streptomyces lincolnensis. | | Veterinary Drugs and Treatments | Lincomycin has dosage forms approved for use in dogs, cats, swine,
and in combination with other agents for chickens. Because clindamycin
is generally better absorbed, more active, and probably less
toxic, it has largely supplanted the use of lincomycin for oral and
injectable therapy in small animals, but some clinicians believe that
clindamycin does not offer enough clinically significant improvements
over lincomycin to justify its higher cost. For further information,
refer to the Pharmacology or Doses sections. |
| | Lincomycin Preparation Products And Raw materials |
|