- Ceftiofur
-
- $0.00 / 10mg
-
2024-04-10
- CAS:80370-57-6
- Min. Order: 10mg
- Purity: 90%+
- Supply Ability: 10g
- Ceftiofur
-

- $0.00 / 1Kg
-
2024-04-09
- CAS:80370-57-6
- Min. Order: 1Kg
- Purity: 99%
- Supply Ability: 10kg
- ceftiofur
-

- $0.00 / 5KG
-
2024-03-12
- CAS:80370-57-6
- Min. Order: 5KG
- Purity: 93% HPLC
- Supply Ability: 5 TON
|
| | Ceftiofur Chemical Properties |
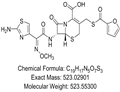
| Melting point | >212°C (dec.) | | density | 1.81±0.1 g/cm3(Predicted) | | storage temp. | 0-6°C | | solubility | DMSO (Slightly), Methanol (Very Slightly, Heated, Sonicated) | | pka | 2.62±0.50(Predicted) | | color | Off-White to Pale Beige | | Stability: | Hygroscopic | | InChIKey | ZBHXIWJRIFEVQY-IHMPYVIRSA-N | | SMILES | N12[C@@]([H])([C@H](NC(/C(/C3=CSC(N)=N3)=N\OC)=O)C1=O)SCC(CSC(C1=CC=CO1)=O)=C2C(O)=O |
| | Ceftiofur Usage And Synthesis |
| Brief introduction |
Ceftiofur(80370-57-6) is the veterinary-exclusive third-generation cephalosporins successfully developed by United States in the 1980s. It entered into market for the first time in the United States. Owing to their excellent antibacterial activity and pharmacokinetic characteristics, it has been officially approved by the United States, Canada, Japan and some European countries for being use in the treatment of respiratory therapy of beef cattle, dairy cattle, horses, pigs, and sheep. Its appearance is white to light yellow powder. It is insoluble in water, slightly soluble in acetone, and almost insoluble in ethanol.
| | Antibacterial activity | Ceftiofur has a broad antimicrobial spectrum with strong antibacterial activity with strong antibacterial activity against Gram-positive and Gram-negative bacteria, and some anaerobic bacteria. Yancy RJ et al has studied the 264 pathogen strains from 17 kinds of veterinary clinical bacteria isolated from cattle, pigs, horses, poultry, dogs, and cats. Except S. aureus, the ceftiofur has a stronger antimicrobial activity than ampicillin against almost all strains (including strains production β-lactamase). Salmon S A et al has studied the antibacterial effect of ceftiofur and its major metabolite furan carbonyl ceftiofur on 539 veterinary clinical isolated pathogen strains and has shown that these two drugs has the same or similar antibacterial activity against Gram-negative bacteria.
| | Cephalosporins |
Ceftiofur(80370-57-6) is a kind of animal-specific third-generation cephalosporin antibiotics with broad antibacterial spectrum and strong antibacterial activity. It has strong antibacterial activity against Gram-positive bacteria, Gram-negative bacteria, and anaerobic bacteria with comparable or weaker efficacy as first-generation cephalosporins in treating Gram positive bacteria. It also has strong antibacterial activity against many kinds of Gram-negative bacteria such as E. coli, Salmonella typhi Pasteurella multocida and Pasteurella hemolytica, and Streptococcus, being suitable for treating infection of respiratory, urinary tract and caused by various kinds of susceptible bacteria, especially for the prevention and treatment of the early-stage death of baby chicks caused by E. coli, Salmonella, Pseudomonas aeruginosa and Staphylococcus aureus, E. coli mediated yellow scour of daily old piglet and wound infection caused by cut of the umbilical cord, cutting teeth, and cutting tails as well as the porcine contagious pleuropneumonia and bovine bronchial pneumonia caused by Haemophilus Actinobacillus. It is generally not suitable for the treatment of the cows and goats mastitis. This product has characteristics such as complete intramuscular absorption and a long elimination half-life.
The injection varieties of the third-generation cephalosporins such as cefotaxime, ceftriaxone has a lower efficacy on treating Gram-positive bacteria than the first-generation cephalosporins but has good antibacterial effect against Streptococcus pneumoniae (including penicillin-resistant strains), Streptococcus pyogenes and other kinds of Streptococcus; It has strong antibacterial effect against Escherichia coli, Klebsiella pneumoniae, Proteus bacteria and some other kinds of Gram-negative bacteria; It also has strong effect against Haemophilus influenzae, Neisseria meningitidis, Neisseria gonorrhoeae and Moraxella catarrhalis bacteria while its effect on Serratia, Enterobacter, Acinetobacter and Pseudomonas vary among different bacteria varieties. Drug varieties of anti-Pseudomonas such as ceftazidime, cefoperazone, cefpiramide have a relative poor effect on Gram-positive cocci bacteria while having a comparable antibacterial activity on gram-negative bacilli as other third-generation cephalosporins. It has strong antibacterial activity against Pseudomonas aeruginosa. Most kind of third-generation cephalosporins is highly stable against the broad-spectrum β-lactamase generated by the gram-negative bacillus bacteria.
| | Precautions | 1. Cephalosporins allergic animals should be disabled; animal allergic to penicillin should apply with caution.
2. Application of this drug to the horse under stress conditions can cause acute diarrhea which may be lethal. Once happening, immediately stop and take the appropriate therapeutic measures.
3. Animals of renal insufficiency should pay attention to adjust the dose.
4. Injection-purpose ceftiofur sodium should be dissolved in the water before injection to prepare ceftiofur to a concentration of 50 mg per ml (It can maintain its effect for seven days when stored at 2~8 ℃; it can maintain its effect for 12 hours upon 15~30 ℃ ambient temperature). Use suspension for injection and shake well before use. It is not suitable for been frozen and should be used up within 14 days after the first use.
5. Withdrawal period: injection-purpose ceftiofur sodium: cattle: 3 days; pigs: two days. Ceftiofur hydrochloride suspension: cattle: two days.
The above information is edited by the Chemicalbook of Dai Xiongfeng.
| | Uses | Animal-specific antibiotic medicines used for treating Gram-positive and Gram-negative bacteria and Mycoplasma infection.
Veterinary (original powder)
| | Chemical Properties | Powder. | | Uses | antifungal | | Uses | A third generation cephalosporin antibiotic for used in veterinary medicine. It is resistant to antibiotic resistance enzyme beta-lactamase. | | Side effects | Adverse effects of Ceftiofur were the first day of intramuscular injection and the mare showed no signs of side effects of the drug. However, 24 hours later, during the second application, the patient experienced incoordination, dizziness and loss of balance. At the same time, dexamethasone was given. Signs returned and the mare returned to normal after the reaction. Treatment with ceftiofur was changed to enrofloxacin and the animal recovered completely. | | Safety Profile | When heated to
decomposition it emits acrid smoke and
irritating fumes. |
| | Ceftiofur Preparation Products And Raw materials |
|