| | Hydrogen peroxide Basic information | | Uses |
| | Hydrogen peroxide Chemical Properties |
| Melting point | -33 °C | | Boiling point | 108 °C | | density | 1.13 g/mL at 20 °C | | vapor density | 1.1 (vs air) | | vapor pressure | 23.3 mm Hg ( 30 °C) | | refractive index | 1.3350 | | Fp | 107°C | | storage temp. | 10-30°C | | solubility | diethyl ether: soluble | | pka | 11.5(at 25℃) | | form | Solution | | Specific Gravity | approximate 1.13 | | color | ≤10(APHA) | | PH Range | 6 - 8 at 25 °C | | Odor | Slightly pungent, irritating odor | | PH | 2-4 (H2O, 20°C) | | Water Solubility | miscible | | Merck | 14,4798 | | BRN | 3587191 | | Exposure limits | TLV-TWA 1 ppm (~1.5 mg/m3) (ACGIH), MSHA,andOSHA),IDLH75 ppm(NIOSH). | | Dielectric constant | 84.2(0℃) | | Stability: | Slightly unstable - will very slowly decompose. Decomposition is promoted by catalysts and heating, so store cool. Light sensitive, keep in the dark. May contain stabilizer. Reacts with rust, brass, zinc, nickel, finely powdered metals, copper and iron and their alloys. | | InChIKey | MHAJPDPJQMAIIY-UHFFFAOYSA-N | | LogP | -1.57 at 20℃ | | CAS DataBase Reference | 7722-84-1(CAS DataBase Reference) | | IARC | 3 (Vol. 36, Sup 7, 71) 1999 | | NIST Chemistry Reference | Hydrogen peroxide(7722-84-1) | | EPA Substance Registry System | Hydrogen peroxide (7722-84-1) |
| Hazard Codes | Xn,C,O | | Risk Statements | 22-41-37/38-34-20/22-8-35-5 | | Safety Statements | 26-39-45-36/37/39-28A-17-28-1/2 | | RIDADR | UN 2014 5.1/PG 2 | | WGK Germany | 1 | | RTECS | MX0899500 | | TSCA | Yes | | HS Code | 2847 00 00 | | HazardClass | 5.1 | | PackingGroup | II | | Hazardous Substances Data | 7722-84-1(Hazardous Substances Data) | | Toxicity | LD50 oral (rat)
75 mg/kg (70%)
LD50 skin (rabbit)
700 mg/kg (90%)
LD50 skin (rabbit)
9200 mg/kg (70%)
LC50 inhal (rat)
>2000 ppm (90%)
PEL (OSHA)
1 ppm (1.4 mg/m3) (90%)
TLV-TWA (ACGIH)
1 ppm (1.4 mg/m3) (90%) | | IDLA | 75 ppm |
| | Hydrogen peroxide Usage And Synthesis |
| Uses | Hydrogen peroxide (H2O2) in a purified form is explosive. In a dilute form in water, it is
used as an antiseptic and oxidizing agent. | | Description | Hydrogen peroxide (H2O2) is a strong oxidizing agent that is used
extensively in industry and medicine. It is usually available as
aqueous solutions in concentrations of 3, 30 or 90 percent by
weight. The 3 percent solution is used as a topical antiseptic and
cleansing agent, and as a constituent in mouthwashes, dentifrices
and sanitary lotions; the 30 percent as an effective bleaching agent
and for other industrial uses; and the 90 percent as a vigorous
oxidizer of rocket fuels. The anhydrous form is a colorless, bittertasting liquid with an ozone-like odor. In the absence of stabilizing
agents (e.g., phosphates, tin), hydrogen peroxide solutions are
unstable and decompose upon standing, agitation, exposure to
light, or heating. Hydrogen peroxide reacts vigorously with many
oxidizing as well as reducing agents. Concentrated solutions are
highly caustic to the skin.
In addition to its effectiveness as a bleach, hydrogen peroxide has
proved to be a useful antimicrobial agent. This latter property has
been utilized in some countries as a preservative of milk and whey. | | Chemical Properties | Colorless liquid; pure compound or 90% solution unstable; bitter taste; density 1.463 g/mL; boils at 150.2°C; freezes at –0.43°C; vapor pressure 9.9 torr
at 50°C and 121.5 torr at 100°C; viscosity 1.245 centipoise at 20°C; surface
tension 80.4 dyn/cm at 20°C; miscible with water in all proportions; soluble in
ether; densities of 30%, 70%, and 90% H2O2 solutions are 1.1081, 1.2839 and
1.3867 g/mL, respectively, at 25°C; freezing points at these concentrations are
–25.7°C, –40.3°C and –11.5°C, respectively; and their boiling points are
106.2°C, 125.5°C and 141.3°C, respectively; decomposed by many organic solvents; pKa at 25°C is 11.62. | | Physical properties | Colorless liquid; pure compound or 90% solution unstable; bitter taste; den-sity 1.463 g/mL; boils at 150.2°C; freezes at -0.43°C; vapor pressure 9.9 torrat 50°C and 121.5 torr at 100°C; viscosity 1.245 centipoise at 20°C; surfacetension 80.4 dyn/cm at 20°C; miscible with water in all proportions; soluble inether; densities of 30%, 70%, and 90% H2O2solutions are 1.1081, 1.2839 and1.3867 g/mL, respectively, at 25°C; freezing points at these concentrations are-25.7°C, -40.3°C and -11.5°C, respectively; and their boiling points are106.2°C, 125.5°C and 141.3°C, respectively; decomposed by many organic sol-vents; pKaat 25°C is 11.62. | | History | Hydrogen peroxide was prepared first by Thenard in 1818. It has many industrial applications. Aqueous solutions at varying concentrations are used for bleaching fabrics, silks, furs, feathers and hair; as a dough conditioner; and a bleaching and oxidizing agent in foods; for cleaning metals; as a laboratory reagent for oxidation; as an antiseptic; in sewage and wastewater treatment; and in preparation of inorganic and organic peroxides. An 80% concentrated solution is used in rocket propulsion. | | Uses | Synthesized hydrogen peroxide is approximately 60% H2O2 by weight and is distilled tohigher concentrations and diluted to lower concentrations for intended purposes. Food grade hydrogen peroxide comes in 35% and 50% concentrations. It is usedfor disinfecting purposes and also as an ingredient in cosmetics, shampoos, and medications.Reagent hydrogen peroxide for chemical and medical laboratories has a concentration of 30%.Standard grades of 35%, 50%, 60%, and 70% are used for industrial bleaching. Generalhousehold hydrogen peroxide is 3% H2O2 and 6% is used by beauticians for hair coloring.Very high grades such as 90% are used as oxidizers in rocket propulsion.
Hydrogen peroxide has a number of environmental uses. Hydrogen peroxide has a number of environmental uses. These include water treatment, odorcontrol, oxidation of pollutants, and corrosion control. Hydrogen peroxide is used to removeiron, manganese, and hydrogen sulfide from water supplies and wastewater. The oxidation ofsubstances such as hydrogen sulfide reduces odors. Because H2O2 decomposes into oxygen andwater, it has the added advantage of lowering the biological oxygen demand of wastewater.ese include water treatment, odorcontrol, oxidation of pollutants, and corrosion control. Hydrogen peroxide is used to removeiron, manganese, and hydrogen sulfide from water supplies and wastewater. The oxidation ofsubstances such as hydrogen sulfide reduces odors. Because H2O2 decomposes into oxygen andwater, it has the added advantage of lowering the biological oxygen demand of wastewater.
Hydrogen peroxide is used in chemical synthesis and can function as both an oxidizing andreducing agent. Caro’s acid (H2SO5) is made using H2O2. Peracetic acid (C2H4O3) is producedby reacting acetic acid and hydrogen peroxide and is used as a disinfectant. Solid bleachingagents such as perborates and percarbonates are made using H2O2. It is used in epoxida tionand hydroxylation reactions. Epoxidation reactions involve the breaking of double bondsin alkenes, with the carbons then bonding to the same oxygen atom to form an epoxide ring. | | Uses | Antiinfective,
topical. | | Uses | Hydrogen peroxide is used for bleaching silk, fabrics, feathers, and hairs; in refining oils and fats; for cleaning metals surfaces; as an antiseptic; and in rocket propulsion (90% solution). It is marketed as an aqueous solution of 3-90% by weight. | | Uses | hydrogen peroxide is a bleaching and oxidizing agent, detergent, and antiseptic. It is generally recognized as a safe preservative, germ killer, and skin bleacher in cosmetics. If used undiluted, it can cause burns of the skin and mucous membranes. | | Definition | ChEBI: An inorganic peroxide consisting of two hydroxy groups joined by a covalent oxygen-oxygen single bond. | | Definition | hydrogen peroxide: A colourlessor pale blue viscous unstable liquid,H2O2; r.d. 1.44; m.p. –0.41°C; b.p.150.2°C. As with water, there is considerablehydrogen bonding in theliquid, which has a high dielectricconstant. It can be made in the laboratoryby adding dilute acid to bariumperoxide at 0°C. Large quantitiesare made commercially by electrolysisof KHSO4.H2SO4 solutions. Anotherindustrial process involvescatalytic oxidation (using nickel, palladium,or platinum with an anthraquinone)of hydrogen and waterin the presence of oxygen. Hydrogenperoxide readily decomposes in lightor in the presence of metal ions togive water and oxygen. It is usuallysupplied in solutions designated byvolume strength. For example, 20-volume hydrogen peroxide wouldyield 20 volumes of oxygen per volumeof solution. Although the peroxidesare formally salts of H2O2, thecompound is essentially neutral.Thus, the acidity constant of the ionizationH2O2 + H2O ?H3O+ + HO2–is 1.5 × 10-12 mol dm-3. It is a strongoxidizing agent, hence its use as amild antiseptic and as a bleachingagent for cloth, hair, etc. It has alsobeen used as an oxidant in rocketfuels. | | Preparation | Hydrogen peroxide is commercially produced by autooxidation of ethyl anthraquinol in a solvent such as toluene or ethylbenzene. The product ethyl anthraquinone is reduced by hydrogen over supported nickel or platinum catalyst to regenerate back the starting material, ethyl anthraquinol for a continuous production of H2O2. The reaction steps are:
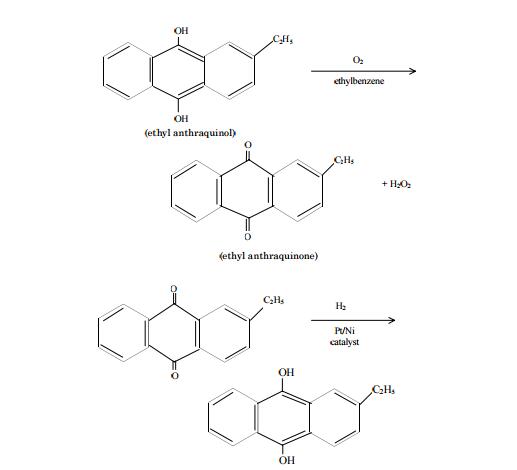
Hydrogen peroxide may also be made by heating 2-propanol with oxygen at 100°C under 10 to 20 atm pressure:
(CH3)2CHOH (CH3)2C(OH)OOH → CH3COCH3 + H2O2
Vapor phase partial oxidation of hydrocarbons also yield H2O2. However, several by-products are generated, the separations of which make the process difficult and uneconomical.
Hydrogen peroxide may also be prepared by treating barium peroxide with dilute sulfuric acid:
BaO2 + 2H2SO4 → H2O2 + BaSO4
Another preparative method involves electrolytic conversion of aqueous sulfuric acid to peroxydisulfate followed by hydrolysis to H2O2 (Weissenstein process). The reaction steps are as follows:
2H2SO4 → H2S2O8 + H2
H2SO5 + H2O → H2SO4 + H2SO5
H2SO5 + H2O → H2O2 + H2SO4
An earlier method, which currently is no longer practiced commercially, involved oxidation of phenyl hydrazine:
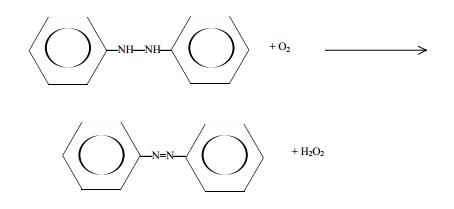 Hydrogen peroxide obtained this way may contain many impurities, depending on the process used. Such impurities are removed by ion exchange, solvent extraction, and distillation. Dilute solutions of H2O2 may be purified
Hydrogen peroxide obtained this way may contain many impurities, depending on the process used. Such impurities are removed by ion exchange, solvent extraction, and distillation. Dilute solutions of H2O2 may be purified
and concentrated by fractional distillation at reduced pressures.
| | Production Methods | From 1920 to 1950, the primary method of production was electrolysis. One process involved passing electric current through sulfuric acid to produce the peroxydisulfate ion (S2O8 2-), which was then hydrolyzed to H2O2: 2H2O + S2O82- (aq) 2H2SO4-(aq) + H2O2(aq).the relatively high cost of electricity of this method encouraged a search for a more economical production process. Hydrogen peroxide is currently produced on a large scale using the anthraquinone autooxidation procedure, which was developed in the 1940s. In this process, an anthraquinone, typically 2-ethyl-anthraquinone, is hydrogenated to a hydroquinone (2-ethyl-anthrahydroquinone) then reoxidized back to the anthraquinone (2-ethyl-anthraquinone) while forming hydrogen peroxide . A metal palladium or nickel catalyst is used to convert the anthraquinone to the hydroquinone, followed by autooxidation in air to generate hydrogen peroxide. The anthraquinone and hydrogen peroxide are separated; the former is recycled to repeat the process while the hydrogen peroxide is purified. | | Reactions | Hydrogen peroxide reacts with many compounds, such as borates, carbonates, pyrophosphates, sulfates, silicates, and a variety of organic carboxylic acids, esters, and anhydrides to give peroxy compounds or peroxyhydrates. A number of these compounds are stable solids that hydrolyze readily to give hydrogen peroxide in solution. | | General Description | A colorless liquid dissolved in water. Vapors may irritate the eyes and mucous membranes. May violently decompose on contact with most common metals and their compounds. Contact with combustible material may result in spontaneous ignition. Corrosive to tissue. Under exposure to fire or heat containers may violently rupture due to decomposition. Used to bleach textiles and wood pulp, in chemical manufacturing, food processing, and in water purification. | | Air & Water Reactions | Water soluble. | | Reactivity Profile | The hazards associated with the use of HYDROGEN PEROXIDE(especially highly concentrated solutions) are well documented. There is a release of enough energy during the catalytic decomposition of 65% peroxide to evaporate all water and ignite nearby combustible materials. Most cellulose materials contain enough catalyst to cause spontaneous ignition with 90% peroxide. Contamination of concentrated peroxide causes the possibility of explosion. Readily oxidizable materials, or alkaline substances containing heavy metals may react violently. Solvents(acetone, ethanol, glycerol) will detonate on mixture with peroxide of over 30% concentration, the violence increasing with concentration. Concentrated peroxide may decompose violently in contact with iron, copper, chromium, and most other metals or their salts, and dust(which frequently contain rust). During concentration under vacuum of aqueous or of aqueous-alcoholic solutions of hydrogen peroxide, violent explosions occurred when the concentration was sufficiently high(>90%), [Bretherick 2nd ed., 1979]. Hydrogen selenide and hydrogen peroxide undergo a very rapid decomposition, [Mellor 1:941(1946-1947)]. | | Hazard | Hydrogen peroxide is a strong oxidizing agent. Concentrated solutions, even a 30% aqueous solution, should be handled carefully. The compound decomposes violently in the presence of trace impurities. Inhibitors are, therefore, added at trace levels to prevent decomposition. Explosion can occur when concentrated solutions are heated or brought in contact with a number of organic substances that are readily oxidizable or that form organic peroxides, such as alcohols, aldehydes, ketones, anhydrides, and carboxylic acids (Patnaik, P. 1999. A Comprehensive Guide to the Hazardous Properties of Chemical Substances, 2nd ed. New York: John Wiley & Sons). Also, reactions with metals, metal alloys, a number of metal salts and oxides, and concentrated mineral acids can proceed to explosive violence. | | Health Hazard | TOXIC; inhalation, ingestion or contact (skin, eyes) with vapors, dusts or substance may cause severe injury, burns or death. Fire may produce irritating and/or toxic gases. Toxic fumes or dust may accumulate in confined areas (basement, tanks, hopper/tank cars, etc.). Runoff from fire control or dilution water may cause pollution. | | Health Hazard | Contact with aqueous concentrations of less than 50% cause skin irritation, but more
concentrated solutions of H202 are corrosive to the skin. At greater than 10%
concentration, hydrogen peroxide is corrosive to the eyes and can cause severe
irreversible damage and possibly blindness. Hydrogen peroxide is moderately toxic
by ingestion and slightly toxic by inhalation. This substance is not considered to
have adequate warning properties.
Hydrogen peroxide has not been found to be carcinogenic in humans. Repeated
inhalation exposures produced nasal discharge, bleached hair, and respiratory tract
congestion, with some deaths occurring in rats and mice exposed to concentrations
greater than 67 ppm | | Health Hazard | Concentrated solutions of hydrogen peroxide are very caustic and can cause burns of skin and mucous membranes. Exposure to its vapors can produce body irritation, lacrimation, sneezing, and bleaching of hair. A dose of 500 mg/kg by dermal route caused convulsions and deaths in rabbits. The oral LD50 value for 90% peroxide solution in mice is 2000 mg/kg.
Oral administration of hydrogen peroxide produced tumors in gastrointestinal tract in mice. There is limited evidence of carcinogenicity in animals. Cancercausing effects of hydrogen peroxide in humans are unknown. Padma and coworkers (1989) reported the promoting effect of hydrogen peroxide on tobacco-specific Nnitrosoamines in inducing tumors in the lung, liver, stomach, and cheek pouch in Syrian golden hamsters and mice. The incidence of cheek pouch tumors increased when peroxide was administered concurrently or applied for a long period after a low initiator dose of N-nitrosamines. . | | Fire Hazard | Hydrogen peroxide is not flammable, but concentrated solutions may undergo
violent decomposition in the presence of trace impurities or upon heating | | Fire Hazard | May explode from friction, heat or contamination. These substances will accelerate burning when involved in a fire. May ignite combustibles (wood, paper, oil, clothing, etc.). Some will react explosively with hydrocarbons (fuels). Containers may explode when heated. Runoff may create fire or explosion hazard. | | Flammability and Explosibility | Hydrogen peroxide is not flammable, but concentrated solutions may undergo
violent decomposition in the presence of trace impurities or upon heating. | | Contact allergens | Hydrogen peroxide is an oxidizing agent used as a topi-
cal antiseptic, and as part of permanent hair-dyes and
color-removing preparations, and as a neutralizing agent
in permanent waving. The concentration of the hydrogen
peroxyde solution is expressed in volume or percentage:
Ten volumes correspond to 3%. It is an irritant. | | Toxicology | Hydrogen peroxide is used as an agent to reduce the number of bacteria in
dairy products or other foodstuffs. In the dairy industry, hydrogen peroxide
also has been used as a substitute for heat pasteurization in the treatment of
milk and as a direct preservative in keeping the quality of the milk. In
Japan, it has been used as a preservative for fish-paste products. Hydrogen
peroxide also has a bleaching effect. The use of highly pure hydrogen peroxide
in manufactured cheese has been approved by the United States Food
and Drug Administration (industrial grade hydrogen peroxide is usually a
3–35% aqueous solution; a commercial home product is a 3% aqueous
solution).
Acute toxicities (LD50) of hydrogen peroxide for rats are 700 mg/kg/b.w.
and 21 mg/kg/b.w. by subcutaneous injection and intravenous injection,
respectively. When large amounts of hydrogen peroxide were injected
directly into the stomachs of rats, weight and blood protein concentrations
were changed slightly. When hydrogen peroxide was mixed with feed, however,
no abnormalities were observed. The use of bactericides has been limited
due to their toxicity to humans, and only hydrogen peroxide currently is
recognized for use. | | Potential Exposure | PotentialExposure:CompoundDescription: Drug,Tumorigen,Mutagen, Human Data; Hormone, PrimaryIrritant (90%); Mutagen, Human Data (20%). Hydrogenperoxide is used in the manufacture of acetone, antichlor,antiseptics, benzoyl peroxide, buttons, disinfectants, phar-maceuticals, felt hats, plastic foam, rocket fuel, sponge rub-ber, and pesticides; as a food and feed additive; flavor; as apackaging material; in bleaching bone; feathers, flour, fruit,fur, gelatin, glue, hair, ivory, silk, soap, straw, textiles, wax,and wood pulp; and as an oxygen source in respiratory pr0-tective equipment. Other specific occupations with potentialexposure include liquor and wine agers, dyers, electropla-ters, fat refiners, photographic film developers, wool prin-ters, veterinarians, and water treaters. | | First aid | If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek med-ical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from ex posure,begin rescue breathing (using universal precautions, includ-ing resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medi-cal attention. If victim is conscious, administer water ormilk. Do not induce vomiting. Medical observation isrecommended for 24- -48 h after breathing overexposure, aspulmonary edema may be delayed. As first aid for pulmo-nary edema, a doctor or authorized paramedic may consideradministering a corticosteroid spray. | | Carcinogenicity | Chronic studies in mice found adenomas
and carcinomas of the duodenum after oral
administration. The IARC has determined that
there is limited evidence in experimental
animals for the carcinogenicity of hydrogen
peroxide and inadequate evidence in humans. | | storage | Use extreme care when carrying out
reactions with hydrogen peroxide because of the fire and explosion potential
(immediate or delayed). The use of safety shields is advisable, and is essential for
experiments involving concentrated (>50%) solutions of hydrogen peroxide. Sealed
containers of hydrogen peroxide can build up dangerous pressures of oxygen, owing
to slow decomposition. | | Shipping | Hydrogen peroxide, stabilized, or hydrogen per-oxide aqueous solutions, stabilized with > 60% hydrogenperoxide,requirea shipping label of “OXIDIZER,CORROSIVE." It falls in Hazard Class 5.1 and PackingGroup I.Hydrogen peroxide, aqueous solutions with > > 40% but not>60% hydrogen peroxide (stabilized as necessary), requiresa shipping label of“OXIDIZER, CORROSIVE." It falls inHazard Class 5.1 and Packing Group II.Hydrogen peroxide, aqueous solutions with not < 20% butnot >40% hydrogen peroxide (stabilized as necessary), requires a shipping label of“OXIDIZER, CORROSIVE."" Itfalls in Hazard Class 5.1 and Packing Group II.Hydrogen peroxide, aqueous solutions with not < <8% but<20% hydrogen peroxide (stabilized as necessary), requiresa shipping label of“OXIDIZER" It falls in Hazard Class5.1 and Packing Group III. | | Purification Methods | The 30% material has been steam distilled using distilled water. Gross and Taylor [J Am Chem Soc 72 2075 1950] made 90% H2O2 approximately 0.001M in NaOH and then distilled it under its own vapour pressure, keeping the temperature below 40o, the receiver being cooled with a Dry-ice/isopropyl alcohol slush. The 98% material has been rendered anhydrous by repeated fractional crystallisation in all-quartz vessels. EXPLOSIVE IN CONTACT WITH ORGANIC MATERIAL. | | Incompatibilities | Contact with many organic compounds can lead to immediate fires or violent
explosions (consult Bretherick for references and examples). Hydrogen peroxide
reacts with certain organic functional groups (ethers, acetals, etc.) to form peroxides,
which may explode upon concentration. Reaction with acetone generates explosive
cyclic dimeric and trimeric peroxides. Explosions may also occur on exposure of
hydrogen peroxide to metals such as sodium, potassium, magnesium, copper, iron,
and nickel. | | Waste Disposal | Excess hydrogen peroxide and waste material containing this substance should be
placed in an appropriate container, clearly labeled, and handled according to your
institution's waste disposal guidelines. For more information on disposal procedures,
see Chapter 7 of this volume. |
| | Hydrogen peroxide Preparation Products And Raw materials |
| Raw materials | Sulfuric acid-->Potassium carbonate-->Potassium hydroxide-->Nitrogen-->Isopropyl alcohol-->Hydrogen-->Phosphoric acid-->Aluminum oxide-->Oxygen-->Ammonium persulfate-->Ammonium sulfate-->Anthraquinone-->Ammonium nitrate-->Potassium persulfate-->Aluminium-nickel-->Ammonium bisulfate-->Bis(2-ethylhexyl) phosphate-->Heavy aromatics-->ALUMINUM OXIDE,ACTIVATED,NEUTRAL,FOR COLUMN CHROMATOGRAPHY,63-200ΜM--> Tris(2-ethylhexyl) phosphate-->2-Ethyl anthraquinone-->DIHYDROTERPINEOL-->AROMATICS | | Preparation Products | 6-Hydroxypicolinic acid-->Sodium perborate-->2-Pyridinol-1-oxide-->Cadmium sulfate-->Cadmium acetate-->polyferric phophat sulfate-->tert-Butyl peroxyacetate-->Erythritol-->Calcium peroxide-->1-Hydroperoxycyclohexyl-1-hydroxycyclohexyl peroxide-->2,6-DIAMINO-3-BROMOPYRIDINE-->Di-(2-ethylhexyl)peroxydicarbonate-->SORBITAN TRIOLEATE-->2-Bromo-3-hydroxy-6-methylpyridine-->Trimethylamine N-oxide-->D-Arabinpyranose-->N,N-Diethylhydroxylamine-->castor oil polyoxyethylene (90) ether-->dodecyl phenyl polyoxyethylene (12) ether-->3-ISOPROPYLPHENOL-->ISOQUINOLINE N-OXIDE-->Sodium pyroantimonate-->2,3-Dimethylpyridine-N-oxide-->3-Bromo-2,6-diaminopyridine ,95%-->3,5-DIBROMOSULFANILAMIDE-->modified soybean phospholipids-->3-METHOXYCATECHOL-->Polyethylene glycol octadecyl ether-->DIHYDROXYTARTARIC ACID-->Thiomorpholine-1,1-dioxide-->trans,trans-2,4-Decadien-1-al-->TRANS-1,2-CYCLOHEXANEDIOL-->LDAO-->Urea hydrogen peroxide-->emulsifier SOPE-6-->OXYCARBOXIN-->PHYSOSTIGMINE-->DIPHENYLCARBAZONE-->DODECANEDIOIC ACID MONOMETHYL ESTER |
|