| Company Name: |
Alfa Chemistry
|
| Tel: |
1-516-6625404 |
| Email: |
support@alfa-chemistry.com |
| Products Intro: |
Product Name:Niridazole
CAS:61-57-4
Package:1g;10g;100g;1KG;5KG
|
|
| | niridazole Basic information |
| Product Name: | niridazole | | Synonyms: | niridazole;Ambilhar;BA32644;Nitrothiamidazole;NSC-136947;1-(5-nitrothiazol-2-yl)imidazolidin-2-one;1-(5-NITRO-2-THIAZOLYL)-2-IMIDAZOLIDINONE;1-(5-nitro-1,3-thiazol-2-yl)imidazolidin-2-one | | CAS: | 61-57-4 | | MF: | C6H6N4O3S | | MW: | 214.204 | | EINECS: | 2005126 | | Product Categories: | | | Mol File: | 61-57-4.mol | 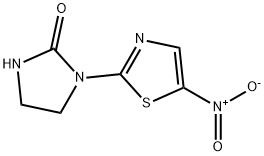 |
| | niridazole Chemical Properties |
| Melting point | 260-262° | | density | 1.561 (estimate) | | refractive index | 1.6200 (estimate) | | form | solid | | pka | 12.73±0.20(Predicted) | | color | Yellow crystals from DMF/MeOH | | Water Solubility | 0.13g/L(25 ºC) | | IARC | 2B (Vol. 13, Sup 7) 1987 | | EPA Substance Registry System | Niridazole (61-57-4) |
| | niridazole Usage And Synthesis |
| Description | Niridazole is a yellow, crystalline solid.
It is almost insoluble in water and most organic
solvents, but it is soluble in dimethylformamide. | | Uses | Antischistosomal. | | Definition | ChEBI: Niridazole is a C-nitro compound and a member of 1,3-thiazoles. | | Indications | Niridazole can be used against blood flukes,
especially Schistosoma haematobium. Tolerance
and efficacy are reduced in S. mansoni
and especially in S. japonicum infections. It is
also used in Dracunculus infections. Immunosuppression,
vomiting, cramps, dizziness, and
headache are among the frequent adverse reactions. | | Mechanism of action | Niridazole causes a depletion of glycogen
in schistosomes by inducing a reduced rate of
conversion of active glycogen phosphorylase
to its inactive form. This is achieved through
inhibition of the enzyme phosphorylase phosphatase,
which normally inactivates glycogen
phosphorylase. It is possible that the active moiety
is not niridazole, but its 5-imino analog,
which can be formed by schistosomes in vitro
under anaerobic conditions . | | Pharmacology | Niridazole exhibits schistosomicide and amebicidal action. The mechanism of action is not
known. It seems likely that it is concentrated in the parasite organism, which causes inhibi�tion of phosphorylase activation, which is expressed in the depletion of glycogen reserves.
It also may inhibit spermatogenesis of parasites by affecting the production of eggs. It is
used for diseases caused by Dracunculus meddinesis, as well as Shistosoma haematobium
and Shistosoma mansoni. It belongs to a group of tertiary drugs and is used only in the
absence of the drug of choice. Synonyms of this drug are ambilhar and others. | | Side effects | Occasional adverse reactions are diarrhea,
electrocardiographic changes, rash, insomnia,
and paresthesia. Psychosis, hemolytic
anemia, and convulsions are rare. Contraindications
are impaired liver function, glucose-6-
phosphatedehydrogenase deficiency, epilepsy,
and severe heart diseases. | | Safety Profile | Confirmed carcinogen with experimental carcinogenic and neoplastigenic data. Poison by intraperitoneal route. Moderately toxic by ingestion. Experimental reproductive effects. Human mutation data reported. Used as an amoebicide and schistosomicidal agent. | | Synthesis | Niridazole, 1-(5-nitro-2-thiazolyl)-2-imidazolidinone (38.1.11), is made by
reacting 2-amino-5-nitrothiazol with 2-chloroethylisocyanate to make the disubstituted
urea (38.1.10). Heating this compound results in an intramolecular N-alkylation reaction
to form the desired imidazolidine derivative, niridazole. 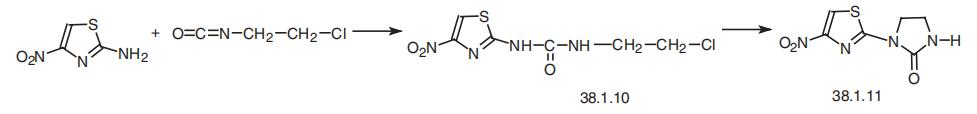
|
| | niridazole Preparation Products And Raw materials |
|