- Cuprous iodide
-
- $6.00 / 1KG
-
2024-03-29
- CAS:7681-65-4
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: g-kg-tons, free sample is available
- Cuprous iodide
-

- $40.00 / 1kg
-
2023-09-07
- CAS:7681-65-4
- Min. Order: 1kg
- Purity: 0.99
- Supply Ability: 10 tons
- Cuprous iodide
-

- $0.00 / 1KG
-
2023-09-06
- CAS:7681-65-4
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 500000kg
|
| | Cuprous iodide Chemical Properties |
| Melting point | 605 °C (lit.) | | Boiling point | 1290 °C/1 atm (decomp)(lit.) | | density | 5.62 g/mL at 25 °C (lit.) | | vapor pressure | 10 mm Hg ( 656 °C) | | refractive index | 2.346 | | Fp | 1290°C | | storage temp. | Store below +30°C. | | solubility | dilute aqueous acid: insoluble(lit.) | | form | Powder | | Specific Gravity | 5.62 | | color | White to Pale Brown | | Water Solubility | INSOLUBLE | | Sensitive | Air & Moisture Sensitive | | Crystal Structure | Cubic, Sphalerite Structure - Space Group F(-4)3m | | Merck | 14,2662 | | Solubility Product Constant (Ksp) | pKsp: 11.9 | | Exposure limits | ACGIH: TWA 1 mg/m3; TWA 0.01 ppm
NIOSH: IDLH 100 mg/m3; TWA 1 mg/m3 | | InChIKey | LSXDOTMGLUJQCM-UHFFFAOYSA-M | | CAS DataBase Reference | 7681-65-4(CAS DataBase Reference) | | NIST Chemistry Reference | Copper(I) iodide(7681-65-4) | | EPA Substance Registry System | Cuprous iodide (7681-65-4) |
| | Cuprous iodide Usage And Synthesis |
| Physical and Chemical Properties | Chemical formula is CuI. The molecular weight is 190.45. White cubic crystal or white powder, toxic. The relative density is 5.62, melting point is 605 °C, boiling point is 1290 °C. Stable to light and air. Cuprous iodide is almost insoluble in water and ethanol, soluble in liquid ammonia, dilute hydrochloric acid, potassium iodide, potassium cyanide or sodium thiosulfate solution, can be c decomposed by oncentrated sulfuric acid and concentrated nitric acid.

The acidic solution of copper sulfate is added excess potassium iodide or under stirring, a mixed solution of potassium iodide and sodium thiosulfate was added dropwise to a solution of copper sulfate, to obtain precipitation of cuprous iodide. In addition to general purpose use as reagents, etc., but also can be used as power-iodide thermal paper conductive layer material, medical sterilization, mechanical bearing temperature agent, but also used for analysis of trace mercury.
Toxicity: Prolonged and repeated contact with the body is harmful, should avoid direct contact with the body. Ingestion is great harm to the body. | | Reaction | Copper catalyst/base admixture useful for screening reactions involving the N-arylation of nitrogen-containing heterocycles.
| | Cuprous halide | Cuprous Iodide and cuprous chloride, cuprous bromide are three common cuprous halides, three are white solid, the photonasty of cuprous iodide is worse than CuBr and CuCl, in light decomposition and precipitation of iodine. In nature, it exists in marshite. Cuprous chloride and cuprous bromide can be produced by the reaction of the corresponding acidic solution of divalent copper salt with copper. Cuprous Iodide was prepared by adding Cu2 + into potassium iodide solution.
Cuprous iodide and Magnesium bromide or lithium phenyl in ether can produce copper phenyl.
The reaction of methyl lithium with cuprous iodide at-15 ℃ or by the reaction of copper nitrate and tetramethyl lead in ethanol at-60 ~-40 ℃ can produce methyl copper.
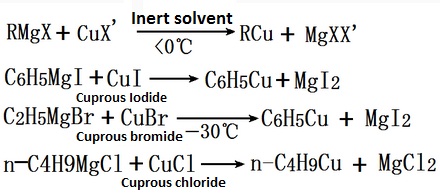
Grignard reagent reacts with cuprous halide to produce cuprous hydrocarbyl, cuprous halide may be cuprous iodide, cuprous bromide, cuprous chloride.
The above information is edited by the chemicalbook of Yan Yanyong. | | Detecting mercuric test by cuprous iodide | Mercury is widely used in various types of industrial instruments, mirror, chemical and metallurgy. West Lik Sang, Saili scattered, Gu Ren music students and other organic mercury common are used in agriculture as seed disinfectants and insecticides. In pharmaceutical, Mercury preparation and the use of mercury Mercuric chloride, calomel, mercury and other benefits withdrawal are used as disinfection, germicides, mercury also can make treatment medication such as diuretics.
And mercury compounds are toxic, such as eating and careless use can cause poisoning.
[principle] After the sublimation of mercury, then it interacts with copper iodide, generating red complex of mercury iodide. This method can be detected 3μg mercury.
Detecting mercuric iodide reaction equation: 2Cu2I2 + HgHgCu2I4 + 2Cu
[Reagents] Cotton cuprous iodide: 5g of copper sulfate, 3g of anhydrous ferrous sulfate, dissolved in 100mL of water, were added a solution of iodide (7g of potassium iodide was dissolved in 50mL water) with stirring, centrifuged the resulting cuprous iodide to precipitation of cuprous iodide, after washing, it was stirred into a cloudy liquid, absorbent cotton impregnated with this solution is cloudy dry for spare.
[Operation] Copper is set in test tubes by Leiyin Xi's method, iodide cotton gently cover the spout. The bottom of the tube is in the direct fire heating, if the presence of mercury, cotton turns red, at the same time conduct blank control.
Also sample 1 drop of digestive juice, applying to cotton cuprous iodide, if present of mercury, cotton turns red.
Reference: in Wiesen, high Ru Qin, Jin Xiaomei editor, common chemical food poisoning rapid disposal technologies.
| | Uses | 1. Cuprous iodide is so widely used as catalyst in organic synthesis, resin modifier, artificial rainfall agents, cathode ray tube cover, as well as sources of iodine in iodized salt. In the presence of 1,2-or 1,3-diamine ligand, cuprous iodide can catalyze reaction of aryl bromide, vinyl bromide and brominated heterocyclic compound converting to the corresponding iodide. The reaction is generally in dioxane solvent, and sodium iodide is used as iodide reagents. Aromatic iodide general is more lively than the corresponding chloride and iodide, therefore, iodide can catalyze a series of reactions involved in coupling a halogenated hydrocarbon, for example, Heck reaction, Stille reaction, Suzuki reaction and the Ullmann reaction. In the present of dichloro bis (triphenylphosphine) palladium (II), cuprous chloride and diethylamine, 2-bromo-1-octen-3-ol with 1-Nonyl acetylene coupling reaction to produce 7-sub-8-hexadecene-6-ol.
2. used as a catalyst for organic reactions, cathode ray tube covering, also used as animal feed additives, etc. copper iodide and mercuric iodide may also be used together as the indicator of measuring rising temperature of mechanical bearing.
3. as a catalyst in many reactions involved in the Grignard reagent, the cuprous iodide can also be in dry Wiff rearrangement reaction.
| | Toxicity | GRAS(FDA§184.1265,2000).
| | Using Limited | 0.01%(FDA§184.1265,2000).
| | synthesis | Copper (I) iodide (CuI), can be prepared by heating iodine and copper in concentrated hydriodic acid, HI. Another preparation route involves the precipitation of the salt by mixing aqueous solutions of potassium or sodium iodide with copper sulfate or any soluble copper(II) salt: CuSO4 + 2KI → CuI2 + K2SO4. The unstable CuI2 compound immediately decomposes into iodine and sparingly soluble CuI, releasing I2: 2CuI2 → 2 CuI + I2. CuI is poorly soluble in water (0.00042 g l−1 at 25°C), but it dissolves in the presence of NaI or KI to give the linear complex anion [CuI2]−. Dilution of such solutions with water reprecipitates CuI. In many aspects, copper(I) iodide may be regarded as a subvalent iodide of copper (vide supra).
| | Production method | Derived by the reaction of copper sulfate and potassium iodide under weakly acidic conditions.
| | Chemical Properties | Copper(I) iodide is naturally occurring as the white to reddishbrown mineral marshite. It is essentially insoluble in water but dissolves in complexing media such as ammonia, cyanide, and halide solutions. Copper(I) iodide is manufactured pyrometallurgically by the reaction of hot copper with iodine vapor in a process essentially identical to that for the preparation of copper(I) chloride. It has also been produced by the reaction of copper powder with methanol solutions of iodine at ambient temperatures. Copper(I) iodide is used as a feed additive and as a heat and light stabilizer in certain polymers and photographic emulsions. It is also used in cloud seeding and in the oil-drilling industry as an anticorrosion adjuvant. | | Chemical Properties | Cuprous Iodide, Cu2O, cubic white crystals, practically insoluble in H2O or alcohol, soluble in NH4OH, potassium iodide, or potassium cyanide. Used in Sandmeyer’s reaction to synthesize aryl chlorides. | | Uses | A catalyst for N-arylation of amines and amino acids | | Uses | As catalyst in organic reactions; as ice-nucleating chemical; as coating in cathode-ray tubes; as source of iodine in animal feeds; with HgI2 as tempereture indicator; bactericide. | | Uses | Copper (I) Iodide used in the preparation of alkynyl imines and the synthesis of pyrroles and pyrrole heterocycles. It is also used in the synthesis of BTBT derivatives ([1]Benzothieno[3,2-b]benzothio
phene) for use as semiconductors in transistors. | | General Description | Odorless tan or off-white solid. Sinks in water. | | Reactivity Profile | Cuprous iodide has weak oxidizing or reducing powers. Redox reactions can however still occur. The majority of compounds in this class are slightly soluble or insoluble in water. If soluble in water, then the solutions are usually neither strongly acidic nor strongly basic. These compounds are not water-reactive. | | Hazard | Toxic. | | Health Hazard | Inhalation causes irritation of nose and throat. Ingestion of copper salts produces violent vomiting and purging, intense pain, collapse, coma, convulsions, and paralysis. Contact with eyes or skin causes irritation. | | Fire Hazard | Special Hazards of Combustion Products: Irritating hydrogen iodide or iodine vapors may form in fire. | | Purification Methods | It can be freshly prepared by dissolving an appropriate quantity of CuI in boiling saturated aqueous NaI over 30minutes. Pure CuI is obtained by cooling and diluting the solution with water, followed by filtering and washing sequentially with H2O, EtOH, EtOAc, Et2O and pentane, then drying in vacuo for 24hours [Dieter, J Am Chem Soc 107 4679 1985]. Alternatively wash it with H2O, then EtOH and finally with Et2O containing a little iodine. Traces of H2O are best removed first by heating at 110o and then at 400o. Exess of I2 is removed completely at 400o. It dissolves in Et2O if an amine is present to form the amine complex. On heating it becomes red, then black, but changes to white on cooling. It is sparingly soluble in H2O or alkali iodide solutions but readily soluble in NH3 (which absorbs CO) and in cyanide or thiosulfate solutions. [Glemser & Sauer in Handbook of Preparative Inorganic Chemistry (Ed Brauer) Academic Press Vol II p 1007 1965, Bawn & Ledwith Chem Ind (London) 1180 1957.] |
| | Cuprous iodide Preparation Products And Raw materials |
|