- Moricizine
-

- $15.00 / 1KG
-
2021-07-13
- CAS:31883-05-3
- Min. Order: 1KG
- Purity: 99%+ HPLC
- Supply Ability: Monthly supply of 1 ton
- Moricizine
-

- $15.00 / 1KG
-
2021-07-10
- CAS:31883-05-3
- Min. Order: 1KG
- Purity: 99%+ HPLC
- Supply Ability: Monthly supply of 1 ton
|
| | Moricizine Basic information |
| Product Name: | Moricizine | | Synonyms: | TIMTEC-BB SBB005991;CARBAMIC ACID, [10-[3-(4-MORPHOLINYL)-1-OXOPROPYL]-10H-PHENOTHIAZIN-2-YL]-, ETHYL ESTER;Moricizine;Moricizin;10-(3-Morpholinopropionyl)-10H-phenothiazin-2-yl carbaminoic acid, ethyl ester;10-(3-Morpholinopropionyl)phenothiazine-2-carbamic acid ethyl ester;Ethmozin:Moricizine;N-[10-(3-Morpholinopropionyl)-10H-phenothiazin-2-yl]carbamic acid ethyl ester | | CAS: | 31883-05-3 | | MF: | C22H25N3O4S | | MW: | 427.52 | | EINECS: | 250-854-5 | | Product Categories: | API | | Mol File: | 31883-05-3.mol | 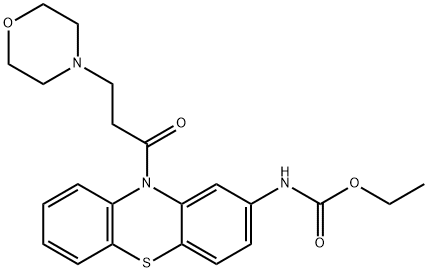 |
| | Moricizine Chemical Properties |
| Melting point | 156-157° | | Boiling point | 625.0±55.0 °C(Predicted) | | density | 1.315±0.06 g/cm3(Predicted) | | storage temp. | -20°C Freezer, Under inert atmosphere | | solubility | Chloroform (Slightly), Methanol (Very Slightly) | | pka | 6.4(at 25℃) | | form | Solid | | color | Pale Orange to Light Orange |
| | Moricizine Usage And Synthesis |
| Originator | Ethmozine,Bristol-Myers Squibb | | Uses | Moricizine, is Phenothiazine (P318040) derivative, which It was used as an Antiarrhythmic agent. It is also shown that moracizine is effective in suppressing premature ventricular contractions, couplets, and nonsustained ventricular tachycardia. | | Uses | Cardiac depressant (anti-arrhythmic). | | Definition | ChEBI: A phenothiazine substituted on the nitrogen by a 3-(morpholin-4-yl)propanoyl group, and at position 2 by an (ethoxycarbonyl)amino group. | | Manufacturing Process | To a solution of 10 g (0.035 mole) of ethyl phenthiazine-2-carbamate in 30 ml
of anhydrous toluene is added dropwise 5.3 g (0.042 mole) of 3-
chloropropionyl chloride, and the mixture is refluxed at 110-120°C for 4
hours, followed by clarifying the mixture with activated carbon and cooling it
to room temperature. A precipitate of ethyl 10-(3-chloropropionyl)-
phenthiazine-2-carbamate is removed by filtration. The yield is 10.2 g (77.5%
of the theoretical amount), M.P. 169-170°C.
10.2 g of ethyl 10-(3-chloropropionyl)-phenthiazine-2-carbamate ester is
dissolved in 50 ml of toluene, 4.72 g of morpholine is added thereto, and the
mixture is refluxed at 110-120°C for a period of 3 hours. A precipitate of
morpholine hydrochloride is removed by filtration, and the filtrate is washed
with water in order to remove excess morpholine, followed by acidulating with
dilute hydrochloric acid to adjust the pH of the filtrate is adjusted at 3. The
acidic aqueous layer is separated, clarified by treatment with activated carbon
and made alkaline until the pH equals 8-9. This procedure yields the free base
of ethyl 10-(β-morpholylpropionyl)-phenthiazine-2-carbamate, M.P. 156-
157°C.
The free base thus obtained is extracted with toluene, the extract is dried over
magnesium sulphate and to the anhydrous toluene solution is added an
anhydrous ethereal solution of hydrogen chloride until the precipitation of the
target compound is complete. This procedure yields 9.53 g (76.2% of the
theoretical amount) of ethyl 10-(β-morpholylpropionyl)-phenthiazine-2-
carbamate hydrochloride. After recrystallization from dichloroethane, the
target compound melts at 189°C. (decomp.). | | Brand name | Ethmozine (Roberts Pharmaceutical). | | Therapeutic Function | Antiarrhythmic | | General Description | Moricizine, ethyl 10-(3-morpholinopropionyl)phenothiazine-2-carbamate (Ethmozine), is aphenothiazine derivative used for the treatment of malignantventricular arrhythmias. It is categorized as a class ICantiarrhythmic agent, blocking the Na+ channel with 1:1stochiometry. The drug has higher affinity for the inactivatedstate than the activated or resting states. It appearsto bind to a site on the external side of the Na channelmembrane. It has been used to suppress life-threateningventricular arrhythmias. | | Clinical Use | Moricizine (Ethmozine) is an antiarrhythmic used to
treat documented life-threatening arrhythmias.
Moricizine is indicated for the treatment of documented
ventricular arrhythmias, particularly sustained ventricular
tachycardia. Moricizine was evaluated in the CAST
II clinical trial for the prevention of postinfarction ventricular
premature complexes. It was ineffective and
found to be proarrhythmic. Patients in the moricizine
arm of the trial exhibited a greater incidence of sudden
cardiac death than did controls. | | Side effects | The principal adverse gastrointestinal effect of moricizine
is nausea (7%). Abdominal discomfort has also
been reported. Dizziness (11%) is the most frequently
reported CNS-related adverse effect. Such reactions
increase in frequency with prolonged drug administration.
As with other antiarrhythmic drugs, moricizine has
proarrhythmic activity, which may manifest as new ventricular
ectopic beats or a worsening of preexisting ventricular
arrhythmias. These effects are most common in
patients with depressed left ventricular function and a
history of congestive heart failure. Cardiovascular effects requiring drug withdrawal include conduction defects,
sinus pauses, junctional rhythm, and A-V block. | | Drug interactions | Clinically significant interactions with moricizine do not
appear to exist. | | Precautions | Patients with preexisting second- or third-degree A-V
block, cardiogenic shock, or drug hypersensitivity should
not be treated with moricizine. |
| | Moricizine Preparation Products And Raw materials |
|