- Cefoperazone
-

- $15.00/ kg
-
2024-04-25
- CAS:62893-19-0
- Min. Order: 1kg
- Purity: 99.912%
- Supply Ability: 10ton
- Cefoperazone
-
- $0.00 / 1kg
-
2024-04-08
- CAS:62893-19-0
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 20tons
- Cefoperazone
-

- $0.00 / 25KG
-
2023-07-04
- CAS:62893-19-0
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 50000KG/month
|
| | Cefoperazone Basic information |
| Product Name: | Cefoperazone | | Synonyms: | (6R,7R)-7-{[(2S)-2-{[(4-ETHYL-2,3-DIOXO-1-PIPERAZINYL)CARBONYL]AMINO}-2-(4-HYDROXYPHENYL)ACETYL]AMINO}-3-{[(1-METHYL-1H-TETRAZOL-5-YL)SULFANYL]METHYL}-8-OXO-5-THIA-1-AZABICYCLO[4.2.0]OCT-2-ENE-2-CARBO XYLIC ACID;CEFOPERAZONE;5-Thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylicacid,7-[[(2R)-2-[[(4-ethyl-2,3-dioxo-1-piperazinyl)carbonyl]aMino]-2-(4-hydroxyphenyl)acetyl]aMino]-3-[[(1-Methyl-1H-tetrazol-5-yl)thio]Methyl]-8-oxo-,(6R,7R)-;(6R,7R)-7-[[(2R)-2-[[(4-ethyl-2,3-dioxo-1-piperazinyl)carbonyl]amino]-2-(4-hydroxyphenyl)acetyl]amino]-3-[[(1-methyl-1H-tetrazol-5-yl)thio]methyl]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid;(6r-(6-alpha,7-beta(r*)))--tetrazol-5-yl)thio)methyl)-8-oxo;-1-piperazinyl)carbonyl)amino)(4-hydroxyphenyl)acetyl)amino)-3-(((1-methyl-1h;cefoperazine;Cefobis:Cefogram | | CAS: | 62893-19-0 | | MF: | C25H27N9O8S2 | | MW: | 645.67 | | EINECS: | 263-749-4 | | Product Categories: | Inhibitors;Pharmaceutical | | Mol File: | 62893-19-0.mol | 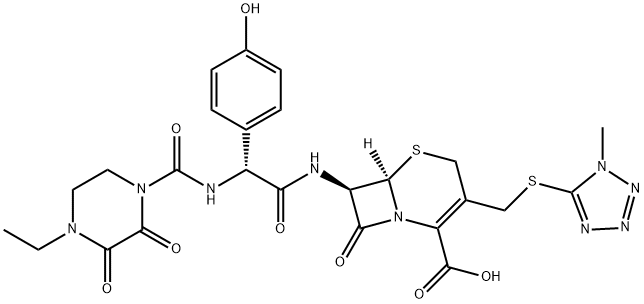 |
| | Cefoperazone Chemical Properties |
| Melting point | 169-171 C | | density | 1.77±0.1 g/cm3(Predicted) | | storage temp. | 2-8°C | | solubility | DMSO (Slightly, Sonicated), Methanol (Slightly, Heated, Sonicated) | | form | Solid | | pka | pKa 2.6 (Uncertain) | | color | White to Off-White | | Merck | 14,1930 | | InChIKey | GCFBRXLSHGKWDP-XCGNWRKASA-N | | SMILES | N12[C@@]([H])([C@H](NC([C@H](NC(N3CCN(CC)C(=O)C3=O)=O)C3=CC=C(O)C=C3)=O)C1=O)SCC(CSC1N(C)N=NN=1)=C2C(O)=O | | CAS DataBase Reference | 62893-19-0(CAS DataBase Reference) |
| | Cefoperazone Usage And Synthesis |
| Description | Cefoperazone has a C-7 side chain reminiscent of piperacillin's and also possesses the C-3 side chain
(MTT ) that often is associated with the bleeding and alcohol intolerance problems among patients taking
cephalosporins. Its useful activity against pseudomonads partly compensates for this, although it is not
potent enough to be used as a single agent against this difficult pathogen. The C-7 side chain does not
convey sufficient resistance to many β-lactamases, although the addition of clavulanic acid or sulbactam
would presumably help. | | Chemical Properties | white crystals | | Originator | Cefobid,Pfizer,W. Germany,1981 | | Uses | Antibacterial. | | Uses | Cefoperazone acid is an antimicrobial β-lactamase inhibitor. | | Uses | Cefoperazone also has a broad spectrum of antimicrobial action, including most clinically
significant microorganisms: Gram-positive, Gram-negative, aerobic, and anaerobic. It is
stable with respect to most beta-lactamases of Gram-positive and Gram-negative bacteria.
Cefoperazone is used for bacterial infections of the lower respiratory tract, urinary and
sexual tracts, bones, joints, skin, soft tissues, abdominal, and gynecological infections.
Synonyms of this drug are cefazon, cefobid, cefobis, and many others. | | Definition | ChEBI: A semi-synthetic parenteral cephalosporin with a tetrazolyl moiety that confers beta-lactamase resistance. | | Manufacturing Process | To a suspension of 3.0 g of 7-[D-(-)-α-amino-p-hydroxyphenylacetamido]-3-
[5-(1-methyl-1,2,3,4-tetrazolyl)thiomethyl]-?3-cephem-4-carboxylic acid in 29
ml of water was added 0.95 g of anhydrous potassium carbonate. After the
solution was formed, 15 ml of ethyl acetate was added to the solution, and
1.35 g of 4-ethyl-2,3-dioxo-1-piperazinocarbonyl chloride was added to the
resulting solution at 0°C to 5°C over a period of 15 minutes, and then the
mixture was reacted at 0°C to 5°C for 30 minutes. After the reaction, an
aqueous layer was separated off, 40 ml of ethyl acetate and 10 ml of acetone were added to the aqueous layer, and then the resulting solution was adjusted
to a pH of 2.0 by addition of dilute hydrochloric acid. Thereafter, an organic
layer was separated off, the organic layer was washed two times with 10 ml of
water, dried over anhydrous magnesium sulfate, and the solvent was removed
by distillation under reduced pressure. The residue was dissolved in 10 ml of
acetone, and 60 ml of 2-propanol was added to the solution to deposit
crystals. The deposited crystals were collected by filtration, washed with 2-
propanol, and then dried to obtain 3.27 g of 7-[D-(-)-α-(4-ethyl-2,3-dioxo)-1-
piperazinocarbonylamino)-p-hydroxyphenylacetamido]-3-[5-(1-methyl-
1,2,3,4-tetrazolyl)thiomethyl]-?3-cephem-4-carboxylicacid, yield 80.7%. The
product forms crystals, MP 188°C to 190°C (with decomposition). | | Brand name | Cefobid (Pfizer). | | Therapeutic Function | Antibiotic | | Antimicrobial activity | A semisynthetic parenteral cephalosporin. It is unstable, losing
activity on storage even at –20°C. A formulation with sulbactam
is available in some countries.
It exhibits moderate activity against carbenicillin-sensitive
strains of Ps. aeruginosa. Activity against Burk. cepacia and
Sten. maltophilia is unreliable. It is much less stable to enterobacterial
β-lactamases than most other cephalosporins of
groups 4–6 and consequently has unreliable activity against
many species, including β-lactamase-producing strains of
H. influenzae
and N. gonorrhoeae. It is active against
Achromobacter, Flavobacterium, Aeromonas and associated
non-fermenters. Past. multocida is extremely susceptible (MIC
<0.01–0.02 mg/L). It exhibits modest activity against most
Gram-negative anaerobes, but not B. fragilis. Sulbactam
increases activity against many, but not all, enterobacteria and
non-fermenters, and almost all B. fragilis.
A 2 g intravenous infusion achieves a peak plasma concentration
of 250 mg/L. The plasma half-life is 1.5–2 h. Over
85% is bound to plasma proteins. It achieves therapeutic concentrations
in tissue and inflammatory exudates. Variable low
levels are found in the sputum up to 1.5% of simultaneous
serum levels. Penetration into CSF is unreliable even in the
presence of meningeal inflammation.
The bile is a major route of excretion, accounting for
almost 20% of the dose. About 20–30% is eliminated in urine,
almost entirely by glomerular filtration. Clearance is effectively
unchanged by renal failure or dialysis.
Side effects associated with the methylthiotetrazole side
chain have been reported. Diarrhea has been notable in some
studies. Marked suppression of fecal flora, with the appearance
of C. difficile, has occasionally been found. There is a 5–10%
incidence of mild transient increases in liver function tests.
Its potential toxicity and the availability of compounds
with better β-lactamase stability and more reliable antipseudomonal
activity have undermined its popularity. | | Synthesis | Cefoperazone, (6R,7R)-7-[(R)-2-(4-ethyl-2,3-dioxo-1-piperazincarboxam�ido)-2-(p-hydroxyphenyl)acetamido]-3-[[(1-methyl-1H-tetrazol-5-yl)thio]methyl]-8-oxo-
5-thia-1-azabicyclo[4.2.0]oct-2-en-2-carboxylic acid (32.1.2.84), is synthesized by acylating
7-amino-3-(1-methyl-1,2,3,4-tetrazol-5-yl)-thiomethyl-3-cefem-4-carboxylic acid (32.1.2.24)
with a mixed anhydride synthesized from ethyl chloroformate and |á-(4-ethylpiperazin-2,
3-dion-1-carbonylamino)-4-hydroxyphenylacetic acid (32.1.2.83), which in turn is synthe�sized from 4-ethylpiperazin-2,3-dion-1-carboxylic acid (32.1.1.29) and the sodium salt of
4-hydroxyphenylglycine. 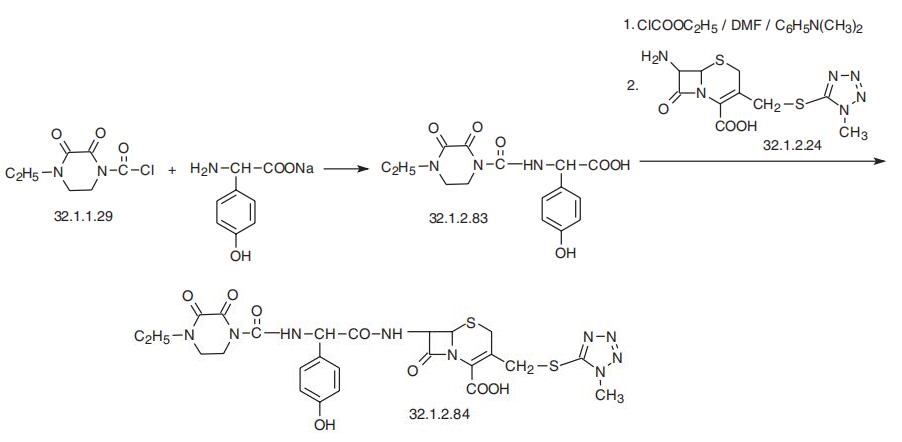
| | references | [1]. kato y1,takahara s,kato s,kubo y,sai y,tamai i,yabuuchi h,tsuji a. involvement of multidrug resistance-associated protein 2 (abcc2) in molecular weight-dependent biliary excretion of beta-lactam antibiotics.drug metab dispos.2008 jun;36(6):1088-96. doi: 10.1124/dmd.107.019125. epub 2008 mar 13.
[2]. craig wa,gerber au. pharmacokinetics of cefoperazone: a review. drugs.1981;22suppl 1:35-45. |
| | Cefoperazone Preparation Products And Raw materials |
|