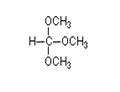
- Trimethyl Orthoformate
-
- $0.00 / 200Kg/Drum
-
2024-04-25
- CAS:149-73-5
- Min. Order: 200KG
- Purity: 99.4
- Supply Ability: 1000kg
- Trimethyl orthoformate
-

- $12.00 / 100mL
-
2024-01-08
- CAS:149-73-5
- Min. Order: 1mL
- Purity: 99%
- Supply Ability: 5000L
Related articles - What is Trimethyl orthoformate?
- Trimethyl orthoformate (TMOF) is the organic compound with the formula HC(OCH?)?. A colorless liquid, it is the simplest ortho....
- Dec 20,2021
|
| | Trimethoxymethane Basic information |
| | Trimethoxymethane Chemical Properties |
| Melting point | -53 °C | | Boiling point | 101-102 °C(lit.) | | density | 0.97 g/mL at 25 °C(lit.) | | vapor density | 3.67 (vs air) | | vapor pressure | 23.5 mm Hg ( 20 °C) | | refractive index | n20/D 1.379(lit.) | | Fp | 60 °F | | storage temp. | Store below +30°C. | | solubility | Miscible with ether, alcohol and benzene. | | form | Liquid | | color | Clear colorless | | explosive limit | 1.4-44.6%(V) | | Water Solubility | 10 g/L (hydrolysis) | | Sensitive | Moisture Sensitive | | Merck | 14,6884 | | BRN | 969215 | | InChIKey | PYOKUURKVVELLB-UHFFFAOYSA-N | | LogP | -0.03-0.09 at 20℃ | | CAS DataBase Reference | 149-73-5(CAS DataBase Reference) | | NIST Chemistry Reference | Methane, trimethoxy-(149-73-5) | | EPA Substance Registry System | Trimethoxymethane (149-73-5) |
| Hazard Codes | F,Xi | | Risk Statements | 11-36 | | Safety Statements | 9-16-26-29 | | RIDADR | UN 3272 3/PG 2 | | WGK Germany | 1 | | RTECS | RM6650000 | | Autoignition Temperature | 255 °C | | TSCA | Yes | | HazardClass | 3 | | PackingGroup | II | | HS Code | 29159080 | | Hazardous Substances Data | 149-73-5(Hazardous Substances Data) | | Toxicity | LD50 orally in Rabbit: 3130 mg/kg |
| | Trimethoxymethane Usage And Synthesis |
| Description | Trimethyl orthoformate is an effective solvent for thallium(III) nitrate-mediated oxidations. It undergoes acid catalyzed reaction with 6-(N-D-ribitylanilino) uracils to form 8-demethyl-8-hydroxy-5-deazariboflavins. | | Chemical Properties | Colorless liquid | | Uses | Trimethyl orthoformate can be used:
- To convert sulfonic acids to methyl esters.
- To convert 2-acylcyclohexanones to the corresponding acetal derivatives.
- To mediate Pinacol reaction of various 1,2-diols with tin(IV) chloride without the formation of water.
- To synthesize 1-substituted-1H-1,2,3,4-tetrazoles via a three-component condensation with amine and sodium azide catalyzed by indium triflate under solvent-free conditions.
- For the N-methylation of amines in the presence of sulfuric acid.
| | Uses | Trimethyl Orthoformate is the most simple orthoester. Used in organic synthesis as a reagent for introducing a protecting group for aldehydes and in the creation of methoxymethylene groups and heterocyclic ring systems. | | Uses | Trimethyl orthoformate is used as a protecting group for aldehydes in organic synthesis, as an additive in polyurethane coatings and as a dehydrating agent in the preparation of surface modified colloidal silica nanoparticles. It is also used as a chemical intermediate in the preparation of vitamin B1 and sulfa drugs. It acts as an effective solvent for thallium(III) nitrate-mediated oxidations. Furthermore. It is utilized for the synthesis of chromone from keto-hydroxy naphthol in the presence of trimethylamine. | | Application | Trimethyl orthoformate was used as dehydrating agent in the preparation of surface-modified colloidal silica nanoparticles.
MOM protection of Diols using Trimethyl Orthoformate
N-Formylation of Amino Acid Esters | | General Description | Trimethyl orthoformate is an effective solvent for thallium(III) nitrate-mediated oxidations. It undergoes acid catalyzed reaction with 6-(N-D-ribitylanilino) uracils to form 8-demethyl-8-hydroxy-5-deazariboflavins. | | Flammability and Explosibility | Highly flammable | | Safety Profile | A skin and eye irritant.
A very dangerous fire hazard when exposed
to heat or flame; can react with oxidizing
materials. Hazardous to prepare. To fight
fire, use CO2, fog, haze. When heated to
decomposition it emits acrid smoke and
irritating fumes. See also ESTERS. | | Synthesis | Trimethyl orthoformate is prepared on an industrial scale by the methanolysis of hydrogen cyanide:
HCN + 3 HOCH3 → HC(OCH3)3 + NH3
Trimethyl orthoformate can also be prepared from the reaction between chloroform and sodium methoxide, an example of the Williamson ether synthesis. | | Precautions | Moisture sensitive. Keep the container tightly closed in a dry and well-ventilated place. Incompatible with acids and strong oxidizing agents. | | References | Protection for the Hydroxyl Group, Including 1,2- and 1,3-Diols
P. G. M. Wuts, in Greene's Protective Groups in Organic Synthesis, 5th ed., ed. by P. G. M. Wuts, John Wiley & Sons, Inc., Hoboken, New Jersey, 2014, Chap. 2, 17.
A Facile Procedure for the Synthesis of N-Formyl Amino Acid Esters
T. Chancellor, C. Morton, Synthesis 1994, 10, 1023. |
| | Trimethoxymethane Preparation Products And Raw materials |
| Raw materials | Sodium hydroxide-->Sodium-->Chloroform | | Preparation Products | N-METHYL-P-ANISIDINE-->N-Boc-5,6,7,8-tetrahydro-3-methoxy-[1,2,4]triazolo[4,3-A]pyrazine ,98%-->6-BROMO-4-CHLOROQUINOLINE-->5,6-DIHYDRO-4-METHOXY-2H-PYRAN-->4,7-DIMETHOXY-1,10-PHENANTHROLINE, 97%-->4-TRIFLUOROMETHYL-N-METHYLANILINE 97-->3-METHOXY-N-METHYLANILINE-->6-bromoquinolin-4(3H)-one-->Pipemidic acid-->Methyl trifluoroacetate-->Methyl methanesulfonate-->Tetramethoxyethylene-->ETHYL 5-AMINO-1-PYRIDIN-2-YL-1H-PYRAZOLE-4-CARBOXYLATE-->Bromoform-->THEBAINE-->2-Methylundecanal dimethylacetal-->(-)-Dimethyl D-tartrate-->P-ANISALDEHYDE DIMETHYL ACETAL-->6,7-DIHYDRO-2-PHENYL-5H-PYRROLO[2,1-C]-1,2,4-TRIAZOLIUM CHLORIDE-->BASIC RED 12 |
|