| | D-METHAMPHETAMINE Basic information |
| Product Name: | D-METHAMPHETAMINE | | Synonyms: | D-METHAMPHETAMINE;DEXTRO-METHAMPHETAMINE;n-methyl-beta-phenylisopropylamine;Norodin;pervertin;Phenethylamine, N,alpha-dimethyl-, (S)-(+)-;stimulex;S(+)-METHAMPHETAMINE | | CAS: | 537-46-2 | | MF: | C10H15N | | MW: | 149.24 | | EINECS: | 208-668-7 | | Product Categories: | API's | | Mol File: | 537-46-2.mol | 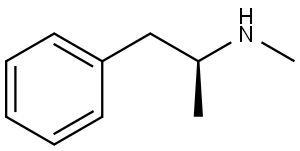 |
| | D-METHAMPHETAMINE Chemical Properties |
| Melting point | 143°C (estimate) | | Boiling point | 230.33°C (estimate) | | density | 0.9285 (estimate) | | refractive index | 1.5105 (estimate) | | Fp | 9℃ | | storage temp. | 2-8°C | | pKa | 10.38±0.10(Predicted) |
| Hazard Codes | F,T | | Risk Statements | 11-23/24/25-39/23/24/25 | | Safety Statements | 16-36/37-45 | | RIDADR | 1851 | | WGK Germany | 1 | | HazardClass | 6.1(b) | | PackingGroup | III | | Hazardous Substances Data | 537-46-2(Hazardous Substances Data) | | Toxicity | A
widely abused drug; a sympathomimetic and central stimulant
that has been used clinically as an anorectic agent. The use of
methamphetamine and related drugs in treating obesity is, however,
now generally discredited. It may cause elevation of
blood pressure, palpitation, and tachycardia, as well as various
CNS effects, including psychotic effects at higher doses.
Chronic use of methamphetamine and related compounds,
especially at high doses as often occurs with drug abuse, can
cause a condition often indistinguishable from schizophrenia.
Abrupt withdrawal may cause fatigue, depression, and sleep
changes. Methamphetamine is an indirect-acting adrenergic
and dopamine agonist that acts principally to release endogenous
catecholamines, thereby increasing their synaptic availability.
Many of its actions can be blocked by adrenergic and
dopamine antagonists. The i.p. LD50 in mice is 70 mg/kg. |
| | D-METHAMPHETAMINE Usage And Synthesis |
| Originator | Desoxyn ,Abbott Laboratories | | Uses | Nasal decongestant. | | Definition | ChEBI: A member of the class of amphetamines in which the amino group of (S)-amphetamine carries a methyl substituent. | | Manufacturing Process | 2 Methods of prepearing of methamphetamine:
1. (-)-Ephedrin was reduced by hydrogenesation with hydrogen in the presence of Pt-C catalyst to give the (+)-N-α-dimethylphenethylamine (methamphetamine), melting point 172°-174°C.
2. Methamphetamine was obtained by the methylation of
phenylisopropylamine.
To give methamphetamine hydrochloride the base methamphetamine was
treated by eqimolar quantity of hydrochloric acid. | | Brand name | Amone;Desoxyn;Dexophrine;Dexoval;Doxyfed;Doxyn
Drinalfa;Efroxine;Euphrodinal;Gardstat;Gerobit;Geronyl;Lemobese;Madrine;Mediatric;Meloda;Metamsustac;Methampex;Methedrinal;Neodrine-triple;Obedrin-la;Obelones;Obe-slim;Pervitin;Phedrisox;Phelantin;Philopon;Soxympamine;Syndrox;Tonedron;Uno. | | Therapeutic Function | Sympathomimetic, Central stimulant | | World Health Organization (WHO) | Metamfetamine, an amfetamine derivative, is controlled under
Schedule II of the 1971 Convention on Psychotropic Substances. See WHO
comment for amfetamine.
(Reference: (UNCPS2) United Nations Convention on Psychotropic Substances (II),
, , 1971) |
| | D-METHAMPHETAMINE Preparation Products And Raw materials |
|