| Company Name: |
Shanghai Daken Advanced Materials Co.,Ltd |
| Tel: |
+86-371-66670886 |
| Email: |
info@dakenam.com |
| Products Intro: |
Product Name:dimethyl (2S,2'S)-1,1'-((2S,2'S)-2,2'-(4,4'-(biphenyl-4,4'-diyl)bis(1H-imidazole-4,2-diyl))bis(pyrrolidine-2,1-diyl))bis(3-methyl-1-oxobutane-2,1-diyl)dicarbamate
CAS:1009119-64-5
Purity:99% Package:100g ;1KG ;5KG 25KG
|
|
|
|
|
- Daclatasvir
-
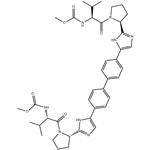
- $35.00 / 1kg
-
2024-03-23
- CAS:1009119-64-5
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: g-kg-tons, free sample is available
- Daclatasvir
-

- $0.00 / 1kg
-
2024-01-17
- CAS:1009119-64-5
- Min. Order: 1kg
- Purity: 99%,single impurity<0.1
- Supply Ability: 1 ton
- Daclatasvir
-

- $0.00 / 100g
-
2023-11-27
- CAS:1009119-64-5
- Min. Order: 100g
- Purity: 99%
- Supply Ability: 500kg
|
| Product Name: | Daclatasvir | | Synonyms: | diMethyl (2S,2'S)-1,1'-((2S,2'S)-2,2'-(4,4'-(biphenyl-4,4'-diyl);Daclatasvir;EBP 883;N,N'-[[1,1'-Biphenyl]-4,4'-diylbis[1H-imidazole-5,2-diyl-(2S)-2,1-pyrrolidinediyl[(1S)-1-(1-methylethyl)-2-oxo-2,1-ethanediyl]]]biscarbamic acid C,C'-dimethyl ester;dimethyl (2S,2'S)-1,1'-((2S,2'S)-2,2'-(4,4'-(biphenyl-4,4'-diyl)bis(1H-imidazole-4,2-diyl))bis(pyrrolidine-2,1-diyl))bis(3-methyl-1-oxobutane-2,1-diyl)dicarbamate;Daclatasvir/EBP883;daclatavir;DiMethyl (2S, 2'S)-1, 1'-((2S, 2'S)-2, 2'-(4, 4'-(biphenyl-4, 4'-diyl)bis(1H-iMidazole-4, 2-diyl))bis(pyrrolidine-2, 1-diyl))bis(3-Methyl-1-oxobutane-2, 1-diyl)dicarbaMate (Related Reference) | | CAS: | 1009119-64-5 | | MF: | C40H50N8O6 | | MW: | 738.88 | | EINECS: | 1592732-453-0 | | Product Categories: | API;Inhibitors | | Mol File: | 1009119-64-5.mol | 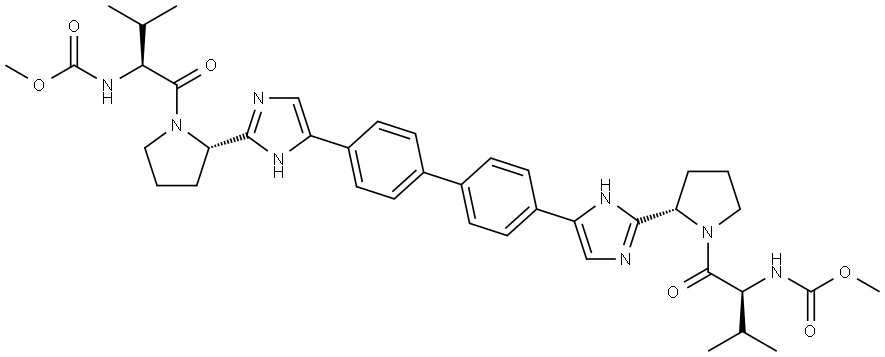 |
| | Daclatasvir Chemical Properties |
| Melting point | >155oC (dec.) | | Boiling point | 1071.2±65.0 °C(Predicted) | | density | 1.249 | | storage temp. | Refrigerator | | solubility | Aqueous Acid (Slightly), Chloroform (Sparingly), DMSO (Sparingly), Methanol (Sparingly) | | form | Solid | | pka | 10.92±0.46(Predicted) | | color | White to Dark Yellow |
| Safety Statements | 24/25 | | HS Code | 29332900 |
| | Daclatasvir Usage And Synthesis |
| Anti-hepatitis C virus drugs | Daclatasvir (Daklinza) has obtained "priority review" status, combined with sorafenib for the treatment of genotype III adult patients with chronic hepatitis C. Daklinza has been the first drug that has been proved of being effective in the treatment of genotype III hepatitis C virus infection without the co-administration with interferon or ribavirin. Interferon and ribavirin are two drugs approved by FDA for the treatment of hepatitis C virus infection. Hepatitis C is a viral disease that can cause inflammation of the liver, resulting in decreased liver function or liver failure. Most patients infected with hepatitis C have no symptoms until liver damage becomes apparent, which may take several years. Globally, genotype III hepatitis C is the second most common genotype of hepatitis C after genotype 1 hepatitis C and is considered to be one of the most refractory genotype diseases. Daklinza is a pan-genotype NS5A replication complex inhibitor, with efficacy of inhibition of RNA replication and viral assembly, dual antiviral effect. For in vitro studies, Daklinza has been demonstrated to have anti-viral effect against genotype 1~6 hepatitis C virus. Daklinza is accompanied by a warning that the combination of amiodarone, Daklinza and Sofosbuvir may cause severe reduced heart rate. Daklinza is an oral tablet with the recommended dose of 60 mg for 1 times/d, in conjunction with Sofosbuvir for a total of 12 weeks.
【Research and development company】 Bristol-Myers Squibb.
【Patent Literature】 WO 2008021927A2 (August 9, 2007).
【Time of marketing】 July 24, 2015 listed in the United States, trade name Daklinza.
【Indications】 Used in combination with Sofosbuvir for the treatment of genotype 3 chronic hepatitis C (HCV) infection.
【Mechanism of action】 HCV nonstructural protein 5A (NS5A) inhibitors. Adverse reactions: headaches and fatigue.
【Formulation and specifications】 Tablets, 30 and 60mg.
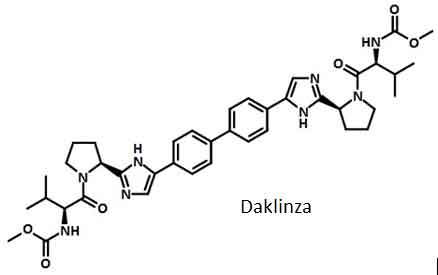
Figure 1 the structure of Daklinza
The above information is edited by the Dongfang of Chemicalbook. (2016-03-03) | | Market status | Daklinza is an inhibitor of hepatitis C virus (HCV) NS5A that is useful in the treatment of genotype 3 chronic hepatitis C infection.
On July 24, 2015, the FDA approved the chronic hepatitis C drug (Bristol-Myers Squibb) for marketing.
The FDA approval process of Daklinza (Bristol-Myers Squibb) has undergone twists and turns. It has been once rejected by the FDA, but finally approved in mid – 2015. The FDA approved the combination of Daklinza and Sofosbuvir for the treatment of hepatitis C gene type 3 patients.
In fact, as early as before the FDA approval, Daklinza had been approved for marketing in Japan, the European Union and South Korea and other countries. In 2014, Japanese health sector approved the application of Daklinza and asunaprevir (Sunvepra) for the treatment of genotype 1 infection. The European Union also approved Daclatasvir to be used in combination with other drugs in the treatment of HCV genotypes 1, 2, 3 and 4 in 2014. Daclatasvir is the first NS5A complex inhibitor approved by European Union (EU). When used in combination with other drugs, compared with the treatment combination of interferon and ribavirin which takes 48 weeks, it has a shorter duration of treatment (12 weeks or 24 weeks).
Daclathavir monotherapy is not recommended, the current mainstream protocol is combination therapy of dacastavir+ sofosbuvir, which is characterized by good efficacy, higher SVR, small side effects and further shortened treatment cycle than other options.
Figure 2 Daclatasvir tablets from United States Bristol-Myers Squibb Company | | Biological activity | Daclatasvir (BMS-790052) is a highly selective HCV NS5A inhibitor with an EC50 of 9-50 pM. It acts on the infectious virus of various HCV replication genotypes and JFH-1 genotype 2a in cell cultures. Phase III; | | In vitro study | BMS-790052 is one of the most potent HCV replication inhibitors reported so far with EC50 values of 50 and 9 pM on HCV genotype 1a and 1b replicons, respectively. BMS-790052 treatment index (CC50/EC50) is 105 or more. It has no effect on the 10 RNA and DNA viruses in group I with EC50 being greater than 10 μM. BMS-790052 is only effective in the treatment of HCV. BMS-790052 acts on the Huh7 cells containing the HCV genotype 1b replicon and BMS-790052 inhibits transient and stable HCV chromosome replication with an EC50 of 1-15 pM. BMS-790052 (100 pM or 1 nM) changes the subcellular localization and biochemical structure of NS5A. BMS-790052 can inhibit the heterozygous replicon containing HCV genotype-4 NS5A gene with an EC50 of 7-13 pM. The NS5A residue 30 in the heterozygous replicon is an important site for selection of resistance to BMS-790052. | | Feature | BMS-790052 is a first-class, highly selective hepatitis C virus (HCV) NS5A inhibitor with an EC50 range at picomolar. | | Uses | Daclatasvir, inhibits the HCV protein NS5A, and thus can be used as a drug candidate for the treatment of hepatitis C (HCV). | | Definition | ChEBI: A member of the class of biphenyls that is a potent inhibitor of nonstructural protein 5A and is used (as its hydrochloride salt) for treatment of hepatitis C. | | Clinical Use | Inhibitor of nonstructural protein 5A (NS5A):
Treatment of chronic hepatitis C infection in
combination with other medication | | Drug interactions | Potentially hazardous interactions with other drugs
Aldesleukin: avoid concomitant use.
Antipsychotics: avoid with clozapine, increased risk
of agranulocytosis. | | Metabolism | Dacarbazine (DTIC) is assumed to be inactive
Dacarbazine is extensively metabolised in the liver Dacarbazine is extensively metabolised in the liver
by the cytochrome P450 isoenzymes CYP1A2 and
CYP2E1 (and possibly in the tissues by CYP1A1)
to its active metabolite 5-(3-methyl-triazen-1-yl)-
imidazole-4-carboxamide (MTIC), which spontaneously
decomposes to the major metabolite 5-amino-imidazole-
4-carboxamide (AIC). About half of a dose is excreted in
the urine by tubular secretion; 50% as unchanged DTIC
and approximately 50% as AIC. |
| | Daclatasvir Preparation Products And Raw materials |
|