|
|
| Product Name: | Silicon Tetrafluoride | | Synonyms: | SILICON TETRAFLUORIDE;SILICIUM TETRAFLUORIDE;Silicon fluoride;SILICON (IV) FLUORIDE;TETRAFLUOROSILANE;SILICON TETRAFLUORIDE, 99.99+%, ELECTRON;Silicon tetrafluoride 99.5%;TETRAFLUOROSILANE: 99.99% | | CAS: | 7783-61-1 | | MF: | F4Si | | MW: | 104.08 | | EINECS: | 232-015-5 | | Product Categories: | | | Mol File: | 7783-61-1.mol | 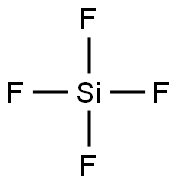 |
| | Silicon Tetrafluoride Chemical Properties |
| Melting point | -90°C | | Boiling point | -86 °C | | density | 1.66 | | vapor pressure | 3517.191kPa at 25℃ | | solubility | reacts with H2O | | form | colorless gas | | color | colorless | | Specific Gravity | 1.66 | | Water Solubility | hydrolyzed by H2O [AIR87] | | Hydrolytic Sensitivity | 8: reacts rapidly with moisture, water, protic solvents | | Merck | 13,8575 | | CAS DataBase Reference | 7783-61-1(CAS DataBase Reference) | | EPA Substance Registry System | Silane, tetrafluoro- (7783-61-1) |
| | Silicon Tetrafluoride Usage And Synthesis |
| Physical and chemical properties | Silicon tetrafluoride is also known as "silyl tetrafluoroethylene." In 1771 it is discovered by the famous Swedish chemist Scheler (Scheele). Chemical formula is SiF4. The molecular weight is 104.08. It is colorless gas with suffocating odor, toxic! Melting point is-90.2 ℃, boiling point is-86℃, sublimation at-95.2℃. The critical temperature is-14.15℃. Critical pressure is 3.715MPa. The relative density is 4.69. It is soluble in ethanol, hydrofluoric acid and nitric acid, insoluble in ether. In humid air it can hydrolyze to produce hydrogen fluoride and silicic acid and form smoke, it can hydrolyze when meets water, and generate orthosilicate H4SiO4 and hydrofluoric acid, hydrofluoric acid which generated by hydrolysis with unhydrolysed silicon tetrafluoride complexed generate fluosilicic . Pure fluorosilicic acid does not exist, it is liable to break down, only can get 60% aqueous solution. The acid of fluosilicic is stronger than sulfuric acid, it is strong acid. It can almost not react with carbon, phosphorus, iodine, and other non-metallic zinc, mercury, hydrogen sulfide, molten potassium chloride, anhydrous alkali metal carbonates and borates. It can stop the magnesium oxide in the air. Potassium and sodium can react in the condition of heating and generate silicon, potassium, sodium of fluoride and the mixture of fluorosilicate. And it can react with aluminum chloride at high temperature to get silicon tetrachloride.

Figure 1 is the molecular structure of silicon tetrafluoride
Preparation method: It can obtain by reaction of concentrated sulfuric acid and calcium fluoride and silica, or by concentrated sulfuric acid decomposing the fluorine-containing phosphate rock. To get extremely pure silicon tetrafluoride, thermal decomposition of fluorosilicate can get: BaSiF6 = BaF2 + SiF4.
Uses: It is used preparation for the organic silicide, fluorine, silicic acid and aluminum fluoride, it can also be used for chemical analysis, oil well drilling, catalysts, fumigation agents. | | Hazardous characteristics | It is non-flammable. It is easy hydrolysis into fluorosilicic acid, hydrogen fluoride forms in the humid air. It is suffocating, irritating and corrosive. It has serious injury to eyes, skin, mucous membranes and respiratory system. Localized corrosion effect is strong, and can cause poisoning decalcification, after a long suffering it can cause disability. Suction gas can cause breathing difficulties, pulmonary edema and chemical pneumonia. Meanwhile visible dental erosion can cause decalcification, chronic fluorine poisoning leads to osteopetrosis. Rats inhale LC50300ppm. After heating the bottle pressure increases, it has the risk of explosion. This information is edited by Chemicalbook Xiaonan (2016-12-01). | | Storage instructions | It is packed in special cylinders, it should be stored in cool, ventilated warehouse. Keep away from heat and sources of ignition. Prevent moisture and rain. Must paste the "gas, corrosive" logo. Aviation, rail transport prohibited. The container can be cooled with water in case of fire, close the valve, cut off the source of fire. Carbon dioxide, dry powder, dry sand to put off fire. Do not use water. | | First-aid measure | When eye contacts it, rinse eye with plenty of water and seek medical advice. Rinse with water when contact with the skin, and then coats with magnesium oxide glycerol ointment, if it has already burned, people should immediately seek medical treatment. When inhales the gas, the patient should be immediately moved to fresh air place, rest and keep warm. Quickly send to hospital for treatment. | | Hazards & Safety Information | Category: harmful gases
Toxicity grading: highly toxic
Acute inhalation toxicity-rat LC50: 2272 PPM
Hazardous characteristics: it can explode when meets heat, sun cylinders can burst; leak emit toxic fumes.
Flammability hazard characteristics: The combustion produces toxic fumes of fluoride
Storage characteristics: Treasury ventilation low-temperature drying; Handle gently.
Extinguishing agent: mist of water, sand
Professional standards: TWA 2.5 mg (fluorine)/cubic meter. | | Chemical Properties | Silicon tetrafluoride exhibits a very pungent odor. Silicon
tetrachloride has a suffocating odor and is moisture sensitive. | | Chemical Properties | A colorless, nonflammable, corrosive and toxic gas. Sharp, pungent suffocating odor, similar to hydrogen chloride. Vapor is heavier than air; density53.6. | | Physical properties | Colorless gas; very pungent odor; fumes heavily in moist air; density of the gas 4.69 g/L; heavier than air, density in air 3.5 (air = 1); sublimes at -95.7°C; solidifies at -90.2°C (under pressure); critical pressure 50atm; decomposes in water forming silicic acid and hydrofluoric acid. | | Uses | Manufacture of fluosilicic acid, intermediate in
manufacture of pure silicon, to seal water out of oil
wells during drilling. | | Preparation | Silicon tetrafluoride is prepared by heating silica with dilute hydrofluoric acid at high temperatures:
SiO2 + 4HF → SiF4 + 2H2O
Also, the tetrafluoride may be obtained by heating the elements:
Si + 2F2 → SiF4. | | General Description | A colorless, nonflammable, corrosive and toxic gas with a pungent odor similar to that of hydrochloric acid. Very toxic by inhalation. Vapor is heavier than air. Under prolonged exposure to heat the containers may rupture violently and rocket. | | Air & Water Reactions | Fumes in air. Decomposed exothermically by water or moisture in the air to hydrofluoric acid and silicic acid [Merck 11th ed. 1989; Handling Chemicals Safely 1980 p. 821]. | | Reactivity Profile | When heated to decomposition SILICON TETRAFLUORIDE emits toxic fluoride fumes [Lewis, 3rd ed., 1993, p. 1138]. Reacts violently with alcohols to form HF. Attacks many metals in the presence of moisture. Reacts violently to explosively with lithium nitride [Porritt, L. J., Chem. Brit., 1979, 15, p. 282]. Mixtures with sodium are shock-sensitive explosives [Leleu, Cahiers, 1975, (79), p. 270]. | | Hazard | Toxic by inhalation, strong irritant to
mucous membranes. | | Health Hazard | TOXIC; may be fatal if inhaled, ingested or absorbed through skin. Vapors are extremely irritating and corrosive. Contact with gas or liquefied gas may cause burns, severe injury and/or frostbite. Fire will produce irritating, corrosive and/or toxic gases. Runoff from fire control may cause pollution. | | Fire Hazard | Some may burn but none ignite readily. Vapors from liquefied gas are initially heavier than air and spread along ground. Some of these materials may react violently with water. Cylinders exposed to fire may vent and release toxic and/or corrosive gas through pressure relief devices. Containers may explode when heated. Ruptured cylinders may rocket. | | Safety Profile | A poison. Moderately toxic by inhalation. A corrosive irritant to skin, eyes, and mucous membranes. When heated to decomposition it emits toxic fumes of F-. See also FLUORIDES and HYDROFLUORIC ACID | | Potential Exposure | Silicon tetrafluoride is not used in industry but may be a discharge byproduct of certain processes, including ore refining and smelting operations. It has been used in the manufacture of silane and fluosilicic acid, and pure electronic silicon. | | Shipping | UN1859 Silicon tetrafluoride, Hazard Class: 2.3; Labels 2.3-Poisonous gas; 8-Corrosive material; Inhalation Hazard Zone B. Cylinders must be transported in a secure upright position, in a well-ventilated truck. Protect cylinder and labels from physical damage. The owner of the compressed gas cylinder is the only entity allowed by federal law (49CFR) to transport and refill them. It is a violation of transportation regulations to refill compressed gas cylinders without the express written permission of the owner. | | Incompatibilities | Water and air reactive Fluorides form explosive gases on contact with strong acids, acid fumes, and sodium. Water and air reactive. Corrosive. Fumes in air. Decomposed exothermically by water, alcohols, or moisture in the air to hydrofluoric acid and silicic acid, forming flammable and potentially explosive hydrogen gas. Attacks many metals in the presence of moisture. | | Waste Disposal | For laboratory quantities, transfer into an evaporating dish containing sodium bicarbonate, spray with ammonia (6M NH4OH)/6 M-ammonium hydroxide/while stirring and then spread with crushed ice. Continue spraying with ammonia until the smoke of ammonium chloride partly subsides and add iced water while stirring. Neutralize and slowly transfer the mixture into a drain with running water. |
| | Silicon Tetrafluoride Preparation Products And Raw materials |
|