| | Pentobarbital Basic information |
| Product Name: | Pentobarbital | | Synonyms: | 5-Ethyl-5-(1-methylbutyl)malonylurea;5-Ethyl-5-(sec-pentyl)barbituric acid;5-ethyl-5-(sec-pentyl)barbituricacid;6(1h,3h,5h)-pyrimidinetrione,5-ethyl-5-(1-methylbutyl)-4;Barbituric acid, 5-ethyl-5-(1-methylbutyl)-;barbituricacid,5-ethyl-5-(2-pentyl);component of Emesert;Dorsital | | CAS: | 76-74-4 | | MF: | C11H18N2O3 | | MW: | 226.27 | | EINECS: | 200-983-8 | | Product Categories: | | | Mol File: | 76-74-4.mol | 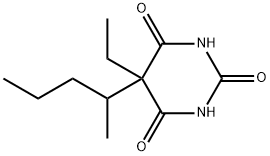 |
| | Pentobarbital Chemical Properties |
| Melting point | 129.5°C | | Boiling point | 367.89°C (rough estimate) | | density | 1.1376 (rough estimate) | | refractive index | 1.4620 (estimate) | | Fp | 9℃ | | storage temp. | 2-8°C | | solubility | Very slightly soluble in water, freely soluble in ethanol. It forms water-soluble compounds with alkali hydroxides and carbonates and with ammonia. | | form | A neat solid | | pka | pK1:8.11(0) (25°C) | | Water Solubility | 679 mg/L | | CAS DataBase Reference | 76-74-4(CAS DataBase Reference) | | NIST Chemistry Reference | Pentobarbital(76-74-4) | | EPA Substance Registry System | Pentobarbital (76-74-4) |
| Hazard Codes | T,F | | Risk Statements | 25-63-39/23/24/25-36/38-23/24/25-11 | | Safety Statements | 7-16-36/37-45-36/37/39-22 | | RIDADR | UN 2811 6.1/PG 3 | | WGK Germany | 3 | | RTECS | CQ5775000 | | HazardClass | 6.1(b) | | PackingGroup | III | | HS Code | 2933530000 | | Hazardous Substances Data | 76-74-4(Hazardous Substances Data) | | Toxicity | A barbiturate that
causes CNS depression, apparently due to a facilitation of
GABA-ergic inhibition. It appears that the site of action of pentobarbital may be the macromolecular complex made up of a
GABA receptor, chloride channel, benzodiazepine-binding site,
and picrotoxin-binding site. Barbiturates have been shown to
compete for dihydropicrotoxinin-binding sites. In clinical use,
barbiturates such as pentobarbital have been largely replaced
as sedative-hypnotics by the much safer benzodiazepines. The
sedative-hypnotic properties of barbiturates may lead to abuse, as
tolerance and dependence are known to occur. In animals, pentobarbital is routinely used for its anesthetic and anticonvulsant
properties, as well as for euthanasia. Pentobarbital, like other
barbiturates, can induce the metabolism of other compounds by
altering cytochrome P450 activity. The oral LD50 in rats is
118 mg/kg. |
| | Pentobarbital Usage And Synthesis |
| Description | Pentobarbital (CRM) (Item No. 20966) is a certified reference material categorized as a barbiturate. Pentobarbital is regulated as a Schedule II compound in the United States. Pentobarbital (CRM) (Item No. 20966) is provided as a DEA exempt preparation. This product is intended for research and forensic applications. | | Chemical Properties | A white or almost white, crystalline powder or colourless crystals | | Originator | Nembutal,Abbott,US | | Uses | Sedative-hypnotic. | | Definition | ChEBI: A member of the class of barbiturates, the structure of which is that of barbituric acid substituted at C-5 by ethyl and sec-pentyl groups. | | Manufacturing Process | Sodium (9.6 parts) was dissolved in butanol (192 parts) and di-n-butyl ethyl 1-methyl-n-butylmalonate (62.8 parts) and urea (14.4 parts) were added to the warm solution with agitation. The mixture was then heated to reflux temperature in three quarters of an hour and maintained for 2 hours. The reaction mass was kept, water (150 parts) added, the aqueous portion separated, and the butanol layer extracted with water (3 x 50 parts). The combined aqueous extracts were then given 3 small extractions with benzene, the aqueous liquors separated, charcoaled, filtered and precipitated with concentrated hydrochloric acid (acid to congopaper). The solid was collected, washed with water, dissolved in N-sodium hydroxide and reprecipitated with carbon dioxide. On recrystallization, from aqueous alcohol, the pentobarbitone was obtained. | | Brand name | Nembutal(Ovation)[Name previously used: Pentobarbitone.];Aethaminalum;Barbityral;Barbopent;Burtylonel;Cafergot p.b.;Calpental;Di-barbs;Dipental;Distonocalm;Ergobel plus;Hypnol;Hypnotal;Hyptonal;Insom rapido;Isoamytal;Isom rapido;Iturate;Narcoren;Nembudeine;Nicaphlogyl;Novarectal;Nova-rectal;Novopentobarb;Novo-pentobarb;Or-trin;Palpent;Pembul;Penbon;Pentalgin;Pentanca;Pentodormol;Pentogen;Pentolos;Petab;Petonel;Praecicalm;Prodormol;Somnophyt;Somnotol;S-spac;Stopp-15;Wans;Wigraine-pb;Yastyl. | | Therapeutic Function | Hypnotic, Sedative | | World Health Organization (WHO) | Pentobarbital is a short-acting barbiturate which is controlled
under Schedule III of the 1971 Convention on Psychotropic Substances. See WHO
comment for barbiturates.
(Reference: (UNCPS3) United Nations Convention on Psychotropic Substances (III),
, , 1971) | | Purification Methods | A solution of the sodium salt in 10% HCl is prepared, and the acid is extracted with ether. Evaporation of the extract gives a solid which is then purified by repeated crystallisation from CHCl3. It sublimes at 95-105o/10-12mm. [Bucket & Sandorfy J Phys Chem 88 3274 1984.] The (+)-and (-)-enantiomers crystallise from 50% aqueous EtOH with m 120-121o and have [�] 25 +4.73o to –4.93o (EtOH) [Kleiderer & Shonle J Am Chem Soc 56 1772 1934]. [Beilstein 24 |
| | Pentobarbital Preparation Products And Raw materials |
|