| Company Name: |
Sinopharm Chemical Reagent Co,Ltd.
|
| Tel: |
86-21-63210123 |
| Email: |
sj_scrc@sinopharm.com |
| Products Intro: |
Product Name:Methicillin Sodium (500 mg) (AS)
CAS:7246-14-2
Package:500 mg
|
METHICILLIN SODIUM (500 MG) (AS) manufacturers
|
| | METHICILLIN SODIUM (500 MG) (AS) Basic information |
| Product Name: | METHICILLIN SODIUM (500 MG) (AS) | | Synonyms: | METHICILLIN SODIUM (500 MG) (AS);Methicillin sodium monohydrate;Methicillin sodium hydrate;SQ-16123;X-1497;Methicillin SodiuM (AS);METHICILLIN SODIUM (500 MG) (AS) USP/EP/BP;Methicillin Sodium (AS) (1410002) | | CAS: | 7246-14-2 | | MF: | C17H19N2O6S.Na.H2O | | MW: | 420.41261 | | EINECS: | | | Product Categories: | | | Mol File: | 7246-14-2.mol | 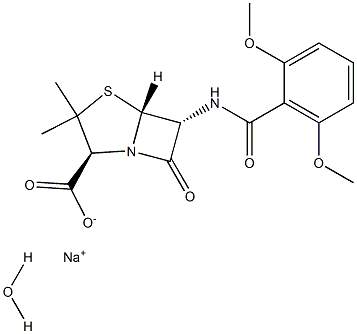 |
| | METHICILLIN SODIUM (500 MG) (AS) Chemical Properties |
| | METHICILLIN SODIUM (500 MG) (AS) Usage And Synthesis |
| Description | Methicillin was synthesized independently by Bristol-Myers Laboratories and Beecham Research Laboratories in 1960. It was the first member of the penicillinase-stable semisynthetic penicillin class of antibiotics to be introduced clinically. This antibiotic is a parenteral penicillin having an antibacterial spectrum similar to that of benzylpenicillin. Although its activity against benzylpenicillin-sensitive bacteria is about 1/3 to 1/50 that of benzylpenicillin, it shows strong activity against benzylpenicillinresistant strains because of its stability toward penicillinase. Methicillin has been used for therapy of respiratory tract and urinary tract infections, sepsis, and gynecological and other infections caused by benzylpenicillin-resistant bacteria. | | Originator | Celbenin ,Beecham,UK,1960 | | Uses | Antibacterial. | | Definition | ChEBI: Methicillin sodium monohydrate is a hydrate. It contains a methicillin sodium. | | Manufacturing Process | To a stirred suspension of 6-aminopenicillanic acid (540 g) in dry alcohol-free chloroform (3.75 liters) was added dry triethylamine (697 ml), and the mixture stirred for 10 minutes at room temperature. It was then cooled in a bath of crushed ice while a solution of 2,6-dimethoxybenzoyl chloride (500 g) in dry alcohol-free chloroform (3.75 liters) was added in a steady stream over 20 minutes. When all the acid chloride had been added the cooling bath was removed and the mixture stirred for 1 hour at room temperature. The mixture was stirred vigorously and sufficient dilute hydrochloride acid (2.3 liters of
0.87 N) was added to give an aqueous layer of pH 2.5. The mixture was
filtered, the layers separated, and only the chloroform layer was retained.
This was stirred vigorously while further dilute hydrochloric acid (0.69 liter of
0.87 N) was added to give an aqueous layer of pH 1. The layers were
separated and again only the chloroform layer was retained. Then the
chloroform layer was stirred vigorously while sufficient sodium bicarbonate
solution (3.2 liters of 0.97 N) was added to give an aqueous layer of pH 6.7 to
7.0. The layers were separated and both were retained. The chloroform layer
was stirred vigorously while sufficient sodium bicarbonate solution (50 ml of
0.97 N) was added to give an aqueous layer of pH 7.7, and again the layers
were separated. The two bicarbonate extracts were combined, washed with
ether (1 liter), and then concentrated at low temperature and pressure until
the concentrate weighed 1,415 g.
The concentrate was treated with dry acetone (22 liters), the mixture well
mixed, and then filtered to remove precipitated solid impurities. Further dry
acetone (4 liters) was added to the filtrate, then the product started to
crystallize slowly. Crystallization was allowed to proceed at a temperature
between 0° and 3°C for 16 hours and then the product (563 g) was collected
by filtration. Dry ether (7.5 liters) was added to the filtrate, and after several
hours a second crop (203 g) of solid was collected. The two crops were
combined to give sodium 2,6-dimethoxyphenylpenicillin monohydrate (766 g,
73%) as a white crystalline solid. | | Brand name | Staphcillin (Apothecon). | | Therapeutic Function | Antimicrobial |
| | METHICILLIN SODIUM (500 MG) (AS) Preparation Products And Raw materials |
|