|
ChemicalBook Optimization Suppliers |
|
| 融点 | 298-300 °C (subl.) (lit.) | | 沸点 | 137.07°C (rough estimate) | | 比重(密度) | 1.62 | | 蒸気圧 | 1.7 mm Hg ( 165 °C) | | 屈折率 | 1.5260 (estimate) | | FEMA | 2488 | FUMARIC ACID | | 闪点 | 230 °C | | 貯蔵温度 | Store below +30°C. | | 溶解性 | 95% ethanol: soluble0.46g/10 mL, clear, colorless | | 外見 | Fine Crystalline Powder | | 酸解離定数(Pka) | 3.02, 4.38(at 25℃) | | 色 | White | | PH | 3.19(1 mM solution);2.57(10 mM solution);2.03(100 mM solution); | | 臭い (Odor) | odorless | | においのタイプ | odorless | | 爆発限界(explosive limit) | 40% | | 水溶解度 | 0.63 g/100 mL (25 ºC) | | Merck | 14,4287 | | JECFA Number | 618 | | BRN | 605763 | | 安定性: | Stable at room temperature. Decomposes at around 230 C. Incompatible with strong oxidizing agents, bases, reducing agents. Combustible. | | InChIKey | VZCYOOQTPOCHFL-OWOJBTEDSA-N | | LogP | -4.02 at 20℃ | | CAS データベース | 110-17-8(CAS DataBase Reference) | | NISTの化学物質情報 | Fumaric acid(110-17-8) | | EPAの化学物質情報 | Fumaric acid (110-17-8) |
| 主な危険性 | Xi | | Rフレーズ | 36 | | Sフレーズ | 26 | | RIDADR | UN 9126 | | WGK Germany | 1 | | RTECS 番号 | LS9625000 | | 自然発火温度 | 375 °C | | TSCA | Yes | | HSコード | 29171900 | | 有毒物質データの | 110-17-8(Hazardous Substances Data) | | 毒性 | LD50 orally in Rabbit: 9300 mg/kg LD50 dermal Rabbit 20000 mg/kg |
| 外観 | 白色の結晶又は結晶性の粉末 | | 性質 | 1. 名称
和名:フマル酸
英名:fumaric Acid
IUPAC名:(2E)-but-2-enedioic acid
2. 分子式
C4H4O4
3. 分子量
116.07
4. 融点
300~302℃(封管中)
5. 溶媒溶解性
に可溶、水に難溶、に不溶。
| | 定義 | 本品は、次の化学式で表されるジカルボン酸である。 | | 溶解性 | 水に難溶。エタノールに可溶。アセトンに微溶。エタノールに溶けやすく、アセトンにやや溶けにくく、水に溶けにくい。 | | 解説 | フマル酸,不飽和の二塩基酸.いろいろな植物中に含まれる.生化学的には,トリカルボン酸サイクルの一員として,コハク酸の脱水素反応により生じる重要な代謝中間体.シス形のマレイン酸と幾何異性の関係にある.マレイン酸の異性化あるいはグリオキシル酸とマロン酸との縮合によって得られる.また工業的には,グルコースのフマル酸発酵によってつくられる.無色の針状結晶.融点287 ℃(封管中).200 ℃ で昇華する.密度1.625 g cm-3.K1 9.3×10-4,K2 10-5(25 ℃).水,エタノールに可溶,エーテル,アセトンに難溶,ベンゼンに不溶.230 ℃ で異性化脱水して無水マレイン酸になる.生体内では,水を付加したリンゴ酸やアンモニアを付加したアスパラギン酸の原料となっている.食品添加物,抗酸剤,合成樹脂や染料の原料に用いられる. | | 用途 | ポリマー類製造時のマレイン酸代替物、食品添加剤(ベーキングパウダー、清涼飲料水剤の酸味料)、抗酸化剤、ゴム、薬品、媒染剤の原料 | | 用途 | 有機合成原料、食品添加物(酸味剤)研究用。 | | 化粧品の成分用途 | pH調整剤、香料 | | 効能 | 乾癬治療薬, 酸味料 | | 説明 | Fumaric acid is an important kind of organic chemical raw materials as well as the intermediate of fine chemical products. Meanwhile, it is also an important kind of derivatives of maleic anhydride, being widely used in food, coatings, resins and plasticizers. In the food industry, fumaric acid, used as souring agent, can be applied to soft drinks, western-style wine, cold drinks, fruit juice concentrate, canned fruit, pickles and ice cream. As an acidic substance used as solid beverage gas production agent, it has excellent bubble durability with delicate product organization.
Fumaric acid has been used as a food acidulant since 1946. As a food additive, it is used as an acidity regulator and can be denoted by the E number E297. Chemically it is an unsaturated dicarbonic acid and is part of the citric acid cycle.
Fumaric acid is a common food additive included in many processed foods to keep them stable and to add tartness. The substance has a more sour flavor than citric acid, another common food additive. Fumaric acid occurs naturally in fumitory, bolete mushrooms, lichen and Iceland moss. As an additive, fumaric acid is produced synthetically, mainly from malic acid from apples. Fumaric acid as an additive is regulated under the Codex Alimentarius General Standard for Food Additives (GSFA), a collection of internationally recognized standards.The U.S. Food and Drug Administration considers it safe. | | 化学的特性 | White, odorless granules or crystalline powder. It is soluble in alcohol, slightly soluble in water and in ether, and very slightly soluble in chloroform.
Fumaric acid is used as a replacement for tartaric acid. It has an odorless, tart, acidic-sour flavor. It may be synthesized by the action of certain fungi (Rhizopus nigricans) on glucose; by oxidation of furfural with sodium chlorate in the presence of vanadium pentoxide. | | 化学的特性 | Fumaric acid is odorless with a tart, acidic-sour favor Fumaric acid is used as a replacement of tartaric acid. | | 化学的特性 | Fumaric acid is a colorless to white, odorless
crystalline powder. Fruity-acidic taste. | | 化学的特性 | Fumaric acid occurs as white, odorless or nearly odorless, granules
or as a crystalline powder that is virtually nonhygroscopic. | | 化学的特性 | Fumaric acid is naturally presented in Corydalis, mushrooms and fresh beef. Product precipitated from the water is monoclinic needle-like, prismatic or leaf-like white crystalline or crystalline powder. It is odorless with a special and strong sour, which is about 1.5 times that of the citric acid. It has a melting point 287 ° C, the boiling point of 290 ° C with subjecting to sublimation at temperature above 200 ° C. When being heated to 230 ° C, it will lose water and become maleic anhydride. Its co-boiling with water can produce DL-malic acid. It is soluble in ethanol, slightly soluble in water and ether, but insoluble in chloroform. The pH value of the 3% aqueous solution is 2.0 to 2.5 with a strong buffering performance, in order to maintain the pH of the aqueous solution at around 3.0. This product is non-toxic; rat-oral LD50: 8000mg/kg. | | 天然物の起源 | Reported found in several plants, Fumaria offcinalis L , Boletus scaber Boll and lean raw fsh | | 使用 | fumaric acid is used to add fragrance to products and to decrease product pH. It can also help keep the pH stable. It is generally used in cleansers. Fumaric acid is naturally occurring in plants, such as lichen and Iceland moss, and in animals. For example, the skin produces fumaric acid when exposed to light. It can also F be synthetically manufactured. | | 使用 | 1. Fumaric acid is used for the production of unsaturated polyester resin. This kind of resin is characterized by excellent resistance to chemical corrosion as well as heat resistance; the copolymer of fumaric acid and vinyl acetate is a kind of excellent adhesive. Its copolymer with styrene copolymer is the raw material for the manufacture of glass fiber. The plasticizer of the fumaric acid is non-toxic and can be applied to the vinyl acetate latex contact with food. This product is the intermediate of pharmaceutical and optical bleaching agents and other fine chemicals. Neutralization of fumaric acid with sodium carbonate can generate sodium fumarate ([17013-01-3]), and then replaced with ferrous sulfate to get iron fumarate, being the drug Fersamal used for the treatment of small red blood cell anemic. The product, as a food additive-sourness agent, used in soft drinks, fruit sugar, jelly, ice cream with most of them used in combination with sourness agent, citric acid. The monosidum salt made from the reaction between fumaric acid and sodium hydroxide can also used as sour seasoning, also used as the intermediate of synthetic resin and mordant.
2. Fumaric acid is included in many dairy-based products. These include dairy drinks such as chocolate milk, cocoa, eggnog, condensed milk and whey protein beverages. It also may be added to clotted cream, milk and cream powders and milk and cream analogues (substitutes). Fumaric acid is added to cheese products, including processed cheese and cheese substitutes. Dairy-based desserts, such as pudding, flavored yogurt, sherbet and sorbet may include fumaric acid as well. Dairy fat spreads and blended spreads can include fumaric acid, and so can preserved eggs and egg-based desserts such as custard.
3. Some processed and packaged foods have fumaric acid added to them to help stabilize them and enhance their flavor. For example, many processed meats, such as bacon and canned meats, have added fumaric acid. Frozen seafood, smoked meats and the edible casings around sausages might also have fumaric acid added to them. Fermented, canned, dried and processed fruits and vegetables can contain the food additive as well. Rice cakes and other precooked rice foods, dried or preserved eggs, mustard, vinegar, cider, wine and other alcoholic beverages are additional examples of foods that might contain fumaric acid. | | 使用 | Occurs in many plants. Essential to vegetable and tissue respiration. Used as an antioxidant. | | 使用 | Fumaric Acid is an acidulant that is a nonhygroscopic, strong acid
of poor solubility. it has a solubility of 0.63 g in 100 ml of distilled
water at 25°c. it dissolves slowly in cold water, but if mixed with
dioctyl sodium sulfosuccinate its solubility improves. the solubility
rate also increases with smaller particle size. a quantity of 0.317 kg
of can replace 0.453 kg of citric acid. it is used in dry
mixes such as desserts, pie fillings, and candy. it is used in dry bever-
age mixes because it is storage stable, free flowing, and nonhygro-
scopic. it functions as a synergistic antioxidant with bha and bht
in oiland lard-base products. in gelatin desserts, it improves the
flavor stability and increases shelf life and gel strength. | | 定義 | Butenedioic Acid: Either of two isomers with the formula HCOOHC:CHCOOH. Both compounds can be regarded as derivatives of ethene in which a hydrogenatom on each carbon has been replaced by a –COOH group. The compounds show cis–trans isomerism.The trans form is fumaric acid (r.d.1.64; sublimes at 165°C) and the cisform is maleic acid (r.d. 1.59; m.p.139–140°C). Both are colourless crystalline compounds used in making synthetic resins. The cis form is rather less stable than the trans form and converts to the trans form at120°C. Unlike the trans form it can eliminate water on heating to form acyclic anhydride containing a–CO.O.CO– group (maleic anhydride).Fumaric acid is an intermediate in the Krebs cycle. | | 定義 | ChEBI: A butenedioic acid in which the C2C double bond has E geometry. It is an intermediate metabolite in the citric acid cycle. | | 調製方法 | Commercially, fumaric acid may be prepared from glucose by the
action of fungi such as Rhizopus nigricans, as a by-product in the
manufacture of maleic and phthalic anhydrides, and by the
isomerization of maleic acid using heat or a catalyst.
On the laboratory scale, fumaric acid can be prepared by the
oxidation of furfural with sodium chlorate in the presence of
vanadium pentoxide. | | 製造方法 | By the action of certain fungi (Rhizopus nigricans) on glucose; by oxidation of furfural with sodium chlorate in the pres- ence of vanadium pentoxide. | | 定義 | Either of two isomers. Transbutenedioic
acid (fumaric acid) is a crystalline
compound found in certain plants. Cisbutenedioic
acid (maleic acid) is used in the
manufacture of synthetic resins. It can be
converted into the trans isomer by heating
at 120°C. | | Biotechnological Production | Currently, fumaric acid is mainly manufactured by chemical synthesis via the
precursor maleic acid, which is produced using either benzene or n-butane via
catalytic oxidation. However, there are enzymatic and fermentative
production routes for fumaric acid. Prior to the advent of inexpensive petroleumbased
chemistry, fumaric acid was produced commercially by fermentation using
organisms of the genus Rhizopus with an annual production of 4,000 metric tons
. Product concentrations from 30 to 130 g.L-1 with yields from 0.3 to 1.0 g
of fumaric acid per gram of glucose and productivities of 0.46–2.0 g.L-1.h-1 have
been reported growing on glucose .
In recent years, new approaches using metabolic engineering have been studied.
For example, fumaric acid concentrations of 28.2 g.L-1 with a productivity of
0.448 g.L-1.h-1 have been reached in fed-batch cultivation of a genetic modified
E. coli . To achieve this result, eight modifications have been implemented.
Fumaric acid could be alternatively synthesized by an enzymatic process
starting from maleic acid as in the chemical synthesis. By whole-cell biocatalysis
of the Pseudomonas alcaligenes strain XD-1, a yield of 0.698 g of fumaric acid per
gram of maleic acid and a production rate of 6.98 g.L-1.h-1 have been reached
. The process has been optimized. The formation of the byproduct malic acid
was avoided due to an inactivation of fumarase by a heat treatment of the cells
beforehand. Finally, a yield of 0.95 g fumaric acid per gram maleic acid and a
production rate of 14.25 g.L-1.h-1 have been observed. | | 一般的な説明 | A colorless crystalline solid. The primary hazard is the threat to the environment. Immediate steps should be taken to limit spread to the environment. Combustible, though may be difficult to ignite. Used to make paints and plastics, in food processing and preservation, and for other uses. | | 空気と水の反応 | Slightly soluble in water. | | 反応プロフィール | Fumaric acid is a carboxylic acid. Carboxylic acids donate hydrogen ions if a base is present to accept them. They react in this way with all bases, both organic (for example, the amines) and inorganic. Their reactions with bases, called "neutralizations", are accompanied by the evolution of substantial amounts of heat. Neutralization between an acid and a base produces water plus a salt. Carboxylic acids with six or fewer carbon atoms are freely or moderately soluble in water; those with more than six carbons are slightly soluble in water. Soluble carboxylic acid dissociate to an extent in water to yield hydrogen ions. The pH of solutions of carboxylic acids is therefore less than 7.0. Many insoluble carboxylic acids react rapidly with aqueous solutions containing a chemical base and dissolve as the neutralization generates a soluble salt. Carboxylic acids in aqueous solution and liquid or molten carboxylic acids can react with active metals to form gaseous hydrogen and a metal salt. Such reactions occur in principle for solid carboxylic acids as well, but are slow if the solid acid remains dry. Even "insoluble" carboxylic acids may absorb enough water from the air and dissolve sufficiently in Fumaric acid to corrode or dissolve iron, steel, and aluminum parts and containers. Carboxylic acids, like other acids, react with cyanide salts to generate gaseous hydrogen cyanide. The reaction is slower for dry, solid carboxylic acids. Insoluble carboxylic acids react with solutions of cyanides to cause the release of gaseous hydrogen cyanide. Flammable and/or toxic gases and heat are generated by the reaction of carboxylic acids with diazo compounds, dithiocarbamates, isocyanates, mercaptans, nitrides, and sulfides. Carboxylic acids, especially in aqueous solution, also react with sulfites, nitrites, thiosulfates (to give H2S and SO3), dithionites (SO2), to generate flammable and/or toxic gases and heat. Their reaction with carbonates and bicarbonates generates a harmless gas (carbon dioxide) but still heat. Like other organic compounds, carboxylic acids can be oxidized by strong oxidizing agents and reduced by strong reducing agents. These reactions generate heat. A wide variety of products is possible. Like other acids, carboxylic acids may initiate polymerization reactions; like other acids, they often catalyze (increase the rate of) chemical reactions. Partial carbonization and formation of maleic anhydride occur at 446° F (open vessel). | | 健康ハザード | Inhalation of dust may cause respiratory irritation. Compound is non-toxic when ingested. Prolonged contact with eyes or skin may cause irritation. | | 燃焼性と爆発性 | Non flammable | | 使用用途 | 1. 殺菌剤としての利用
本化合物は殺菌力を有しているため生鮮食品の殺菌剤として利用されています。 その作用機作は以下の通りです。
◇フマル酸の殺菌剤としての作用機作
- カルボキシル基が非解離の状態で菌体内に取り込まれる。
- 細胞質中でカルボキシル基が解離する事で、細胞質のpHが低下する。
- 上記②の結果、細胞質中の酵素活性が低下し、代謝異常などを誘発する事で菌が死滅する。
2. 食品業界、畜産業界、医療分野での利用例
フマル酸は食品添加物としての安全性が認められており、酸味剤、膨張剤、pH調整剤、調味料などに使用されています。畜産・農業の分野では飼料の添加物、植物の殺菌・殺藻剤として使用されています。また、工業の分野では合成樹脂や染料の原料として利用さています。医療の分野では、フマル酸から作られるフマル酸エステルが乾癬(かんせん)治療に効果があるとして研究が進められています。
3. 生体内での役割
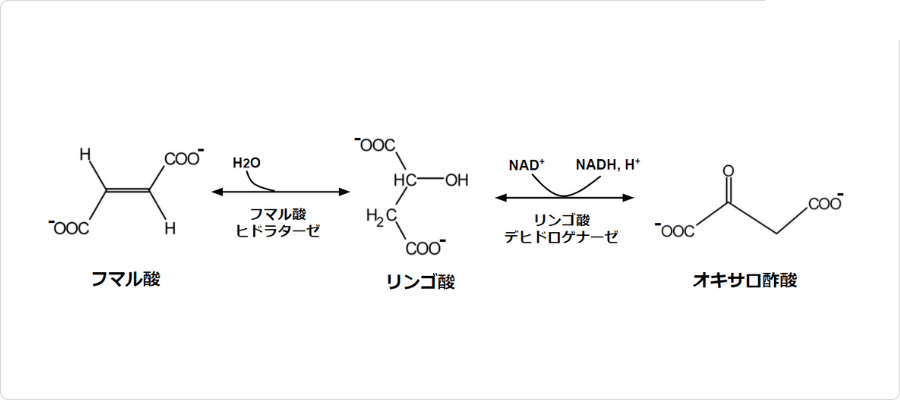
図. クエン酸回路におけるリンゴ酸の反応
フマル酸は酸素呼吸を行う生物のエネルギー生産過程で重要な役割を担っています。具体的には、クエン酸回路において、から生成され、へと変換される中間体として存在しています。
| | 類似物 | フマル酸には幾何異性体が存在します。トランス体がフマル酸であり、シス体がマレイン酸です。
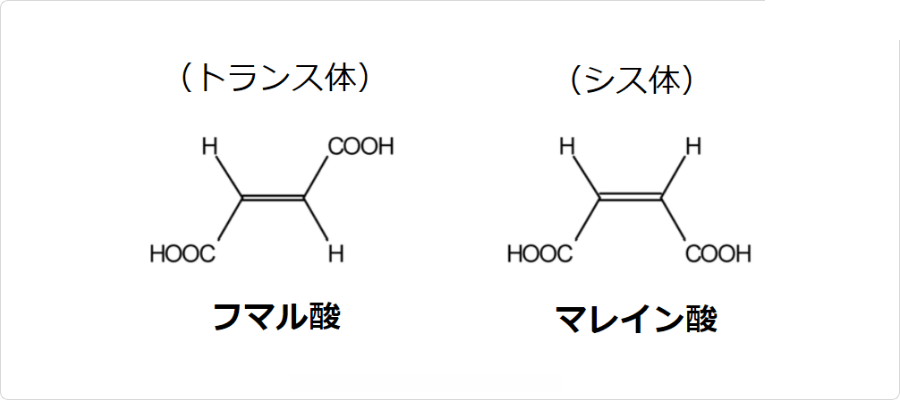
図. フマル酸とマレイン酸の構造
興味深い事に、これらの化合物は化学的性質が大きく異なります。具体的には、トランス体であるフマル酸は、マレイン酸に比べて分子内脱水縮合をしにくく、また、水への溶解度もマレイン酸と比べて非常に低いです。このような性質の違いは、これらの異性体における二つのカルボキシル基の立体的な位置関係で説明できます。その原理については以下の記事で詳しく説明しているので参考になさって下さい。
⇒
| | 応用例(製薬) | Fumaric acid is used primarily in liquid pharmaceutical preparations
as an acidulant and flavoring agent. Fumaric acid may be
included as the acid part of effervescent tablet formulations,
although this use is limited as the compound has an extremely
low solubility in water. It is also used as a chelating agent which
exhibits synergism when used in combination with other true
antioxidants.
In the design of novel pelletized formulations manufactured by
extrusion–spheronization, fumaric acid was used to aid spheronization,
favoring the production of fine pellets. It has also been
investigated as an alternative filler to lactose in pellets.
Fumaric acid has been investigated as a lubricant for effervescent
tablets, and copolymers of fumaric acid and sebacic acid have
been investigated as bioadhesive microspheres.It has been used in
film-coated pellet formulations as an acidifying agent and also to
increase drug solubility.
Fumaric acid is also used as a food additive at concentrations up
to 3600 ppm, and as a therapeutic agent in the treatment of
psoriasis and other skin disorders. | | 安全性プロファイル | Poison by
intraperitoneal route. Mildly toxic by
ingestion and skin contact. A skin and eye irritant. Mutation data reported.
Combustible when exposed to heat or
flame; can react vigorously with oxidizing
materials. When heated to decomposition it
emits acrid smoke and irritating fumes. | | 安全性 | Fumaric acid is used in oral pharmaceutical formulations and food
products, and is generally regarded as a relatively nontoxic and
nonirritant material. However, acute renal failure and other adverse
reactions have occurred following the topical and systemic
therapeutic use of fumaric acid and fumaric acid derivatives in the
treatment of psoriasis or other skin disorders. Other adverse
effects of oral therapy have included disturbances of liver function,
gastrointestinal effects, and flushing.
The WHO has stated that the establishment of an estimated
acceptable daily intake of fumaric acid or its salts was unnecessary
since it is a normal constituent of body tissues.
LD50 (mouse, IP): 0.1 g/kg
LD50 (rat, oral): 9.3 g/kg | | 職業ばく露 | Fumaric acid is used in production of
resins, polyesters, plasticizers, and alkyl surface coatings; as
a food additive; as an antioxidant in resins; to make dyes. | | 応急処置 | If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for atleast 15 min, occasionally lifting upper and lower lids.Seek medical attention immediately. If this chemical contacts the skin, remove contaminated clothing and washimmediately with soap and water. Seek medical attentionimmediately. If this chemical has been inhaled, removefrom exposure, begin rescue breathing (using universalprecautions, including resuscitation mask) if breathing hasstopped and CPR if heart action has stopped. Transferpromptly to a medical facility. When this chemical hasbeen swallowed, get medical attention. Give large quantities of water and induce vomiting. Do not make anunconscious person vomit. | | 発がん性 | No evidence of carcinogenicity
was found in several chronic studies with rats in
which fumaric acid was added to the diet at concentrations
up to 1.5%. As for dermal application, Swiss
mice were treated topically twice weekly with a 1% solution in acetone (volume not specified). Moderate focal
hyperplasia was found in the treated group, but no tumors
developed.
The inhibitory effect of fumaric acid on hepatocarcinogenesis
was examined in male IBR mice fed 0.035% thioacetamide
in the diet for 40 weeks and then fed a basal diet for
48 weeks. The inhibitory effect of 1% fumaric acid in the
basal diet on thioacetamide carcinogenesis was so marked
that no hepatic carcinomas were found in any of the 15 animals
fed fumaric acid in combination with thioacetamide
. Similar inhibitory effects of fumaric acid on
forestomach and lung carcinogenesis in mice (that resulted
from exposure to potassium naphthyridine-3-carboxylate)
have been identified. | | 貯蔵 | Fumaric acid is stable although it is subject to degradation by both
aerobic and anaerobic microorganisms. When heated in sealed
vessels with water at 150–170°C it forms DL-malic acid.
The bulk material should be stored in a well-closed container in a
cool, dry place. | | 純化方法 | Crystallise it from hot M HCl or water and dry it at 100o. [Beilstein 2 IV 2202.] | | 不和合性 | Fumaric acid undergoes reactions typical of an organic acid. | | 不和合性 | Dust cloud from powder or granular
form mixed with air can explode. Incompatible with oxidi zers (chlorates, nitrates, peroxides, permanganates, perchlo rates, chlorine, bromine, fluorine, etc.); contact may cause
fires or explosions. Keep away from alkaline materials,
strong bases, strong acids, oxoacids, epoxides, sulfuric
acid, caustics, ammonia, amines, isocyanates, alkylene oxi des; epichlorohydrin. Decomposes above 350℃ forming
toxic fumes of maleic anhydride. | | 廃棄物の処理 | Use a licensed professional
waste disposal service to dispose of this material. Dissolve
or mix the material with a combustible solvent and burn in
a chemical incinerator equipped with an afterburner and
scrubber. All federal, state, and local environmental regula tions must be observed. | | 規制状況(Regulatory Status) | GRAS listed. Accepted for use as a food additive in Europe.
Included in the FDA Inactive Ingredients Database (oral capsules,
suspensions, syrups, extended release and sustained action chewable
tablets). Included in the Canadian List of Acceptable Nonmedicinal
Ingredients. |
|