- Hypophosphorous Acid
-

- $10.00 / 1kg
-
2024-04-24
- CAS:6303-21-5
- Min. Order: 1kg
- Purity: 99.5
- Supply Ability: 10 ton per month
- Hypophosphorous acid
-

- $10.00 / 1kg
-
2024-04-22
- CAS:6303-21-5
- Min. Order: 1kg
- Purity: 99.99%
- Supply Ability: 1000000000 ton
- Hypophosphorous acid
-

- $30.00 / 1KG
-
2024-01-04
- CAS:6303-21-5
- Min. Order: 1KG
- Purity: 99.9%
- Supply Ability: 20 Tons
|
| Product Name: | Hypophosphorous acid | | Synonyms: | Diphosphoric(IV) acid;Hypophosphorous acid, 50 wt.% solution in water 1LT;Hypophosphorous acid (HPA);Hypophosphorous acid, 50 wt.% solution in H2O;Hypophosphorous Acid, 30 Percent (w/v) Solution;Hypophosphorous acid aqueous solution;Hypophosphorous Acid 50%/70% (HPA);Hypophosphorous acid solution 50 wt. % in H2O | | CAS: | 6303-21-5 | | MF: | HO2P | | MW: | 63.980501 | | EINECS: | 228-601-5 | | Product Categories: | HPA;Inorganics;6303-21-5 | | Mol File: | 6303-21-5.mol | 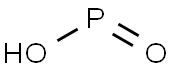 |
| | Hypophosphorous acid Chemical Properties |
| Melting point | -25 °C | | Boiling point | 108 °C (759.8513 mmHg) | | density | 1.206 g/mL at 20 °C(lit.) | | vapor pressure | <17 mmHg ( 20 °C) | | storage temp. | no restrictions. | | solubility | very soluble in H2O, ethanol, ethyl ether | | pka | pK1 1.1. | | form | hygroscopic crystals or colorless oily liquid | | color | Colorless | | Water Solubility | SOLUBLE | | Merck | 13,4894 | | Stability: | Stable. Incompatible with strong bases. Reacts violently with oxidizing agents, strong bases, mercury (II) nitrate and mercury (II) oxide. Do not heat above 100 C. | | InChIKey | GQZXNSPRSGFJLY-UHFFFAOYSA-N | | CAS DataBase Reference | 6303-21-5(CAS DataBase Reference) | | NIST Chemistry Reference | Hypophosphorous acid(6303-21-5) | | EPA Substance Registry System | Phosphinic acid (6303-21-5) |
| | Hypophosphorous acid Usage And Synthesis |
| Outline | Hypophosphorous acid is also known as "hypophosphite" It is colorless oil or deliquescence crystal , it is an important fine chemical product. The main use is as reducing agent for electroless plating, phosphoric prevent discoloration of resins, it can also be used in the esterification reaction catalyst, the refrigerant, in particular for the production of high purity product sodium hypophosphite. There are several methods for preparation, the common industrial method for producing is ion exchange resin method and electrodialysis method.
The chemical properties of hypophosphorous acid, uses, toxicity, and production methods are edited by andy of Chemicalbook. (2016-12-04) | | Chemical properties | It is deliquescent crystals or colorless oil. Melting point: 26.5℃. The relative density (specific gravity): 1.439 (solid, 19℃). It is soluble in water, ethanol and ether, and it can be mixed in any proportion with water, ethanol, acetone. In the air, it easily deliquesce to syrupy liquid, and the aqueous solution is acidic.
Hypophosphorous acid is monobasic acid, in aqueous solution, Hypophosphorous acid is strong acid, Ka = 10-2 (25℃); it is relatively stable at room temperature; disproportionation reaction can proceed at 130℃, decompose into phosphine and phosphorous acid:
2H3PO2=H3PO4+PH3
It has strong reduction, heavy metal salt solution can be restored to metals such as Cu2 +, Hg2 +, Ag +, such as:
4Ag+H3PO2+2H2)=4Ag+H3PO4+4H+
It is weak oxidizer, it can be reduced to phosphine, phosphine when encounters strong reducing agent. | | Uses | 1. Hypophosphorous acid is used as reducing agent for electroless plating;
2. It can be used to prevent discoloration of phosphoric acid resin;
3. It is used as esterification catalyst, the refrigerant;
4. It is used to produce hypophosphite, sodium salts, manganese salts, iron salts are generally used as nourishing substances;
5. Hypophosphorous acid is used in medicine and as reducing agent, the determination of arsenic, tellurium and separation of tantalum, niobium and other reagents.
6. It is strong reducing agent, It can be used for the preparation of sodium hypophosphite, calcium phosphate and other hypophosphite.
7. It can be used for the plating bath. Pharmaceuticals. reducing agent. general reagents.
8. It is strong reducing agent, it can be used in making sodium hypophosphite, calcium phosphate and other hypophosphite.
9. This product is widely used as reducing agent, Ag, Cu, Ni, Hg and other metals are reduced to the corresponding metal, for the verification of As, Nb, Ta and other reagents, it can be used for the preparation of Na, K, Ca, Mn, Fe and other types of hypophosphite. | | Toxicity | It is non-combustible. But when contacts with the hole H agent, it will cause fire. When meets oxidizing agent, violent reaction and combustion can proceed. When it is heated to high, it can decompose into highly toxic phosphine gas, or even explode. It is corrosive. Hypophosphorous acid is often added into soft drinks, and because it is not absorbed. So the risk is small, but particularly strong hypophosphite hurt gastrointestinal. Accidentally it splashes into the eyes or contacts skin, plenty of water is used to washed. Production operators should wear protective clothing and other protective clothing. Production equipment should be sealed, workshop should be ventilated well. | | HAZARDS IDENTIFICATION | Hazard statement:
Causes severe skin burns and eye damage.
Causes serious eye damage
Precautionary statements:
Do not breathe dust/fume/gas/mist/vapors/spray.
Wash thoroughly after handling.
Wear protective gloves and eye/face protection.
IF SWALLOWED: Rinse mouth. Do NOT induce vomiting.
IF ON SKIN (or hair): Remove/Take off immediately all contaminated clothing. Rinse skin with water/shower.
IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if
present and easy to do. Continue rinsing.
IF INHALED: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Immediately call a POISON CENTRE or doctor/physician.
Store locked up.
Dispose of this material and its container to hazardous or special waste collection point. | | Preparation method | 1. Phosphorus and barium hydroxide solution is heated, barium salt Ba (H2PO2) 2 • 2H2O can generate, sulfuric acid is added into hypophosphorous acid barium solution, Ba2+ can precipitate:
Ba(H2PO2)2+H2SO4=BaSO4+2H3PO2
Hypophosphorous acid can be obtained by evaporating under reduced pressure and low temperature crystallization. Due to in this process, the solubility of the barium salt is small, so the concentration of obtained Hypophosphorous acid is not high, industrial product should be purified by recrystallization.
2. the barium oxide (or lime) and solution of white phosphorus is heated together to form secondary barium phosphate (or calcium), and then reacts with sulfuric acid, it is filtered, concentrated to obtain product, or sodium hypophosphite solution proceeds H-type ion exchange resin can derive product. This method requires a large amount of resin, and resin regeneration and washing step is cumbersome, it generally costs more than $ 7 per pound, it is only suitable for small batch production, and not suitable for large-scale industrial applications.
3. Hypophosphorous acid is prepared by electrodialysis method, wherein the electrodialysis cell divides into three parts, they are anode chamber, raw material chamber and cathode chamber, the intermediate is separated by anionic membrane and cationic membrane, between two membranes sodium hypophosphite solution is placed (concentration of 100g/L~500g/L), anode chamber is dilute solution of Hypophosphorous acid 5g/L, anode chamber is dilute sodium hydroxide solution ( 5g /L), between the poles DC (3V~36V) is passed, anode releases oxygen, and generates secondary product of Hypophosphorous acid; cathode emits hydrogen, and generates secondary product of sodium hydroxide, the reaction time is 3~21h. The reactions of anode chamber and cathode chamber are as follows:
anode chamber:
H2O==H++OH-
2OH-==O2+2H2O+4e
H++H2PO2-==H3PO2
cathode chamber:
H2O==H++OH-
2H++2e==H2
Na++OH-==NaOH
Electrodialysis method of preparation Hypophosphorous acid is simple and equipment investment is small, it is suitable for mass production.
4. Starting from the industrial grade sodium hypophosphite, Cl-, SO42-anions which affect the quality indicators of Hypophosphorous acid are removed by precipitation, heavy metal ions are removed from the solution by forming sulfide, and then using strong acid cation exchange resin to obtain sodium secondary phosphate, high purity grade product can obtain. The process can produce high-grade secondary phosphate, technically is feasible, the process is simple, easy operation, good product quality, it can meet the needs of the electronics industry, defense industry and other high-tech fields.
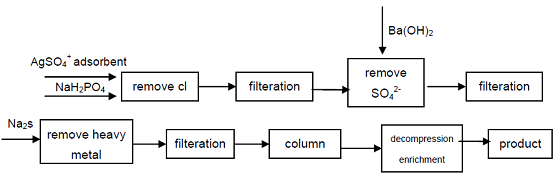
figure 1 Production Process of Hypophosphorous Acid from Industrial Sodium Hypophosphite.
5. Ion exchange resin method: about 70g of cation exchange resin wetted with water is packed into a glass tube with 5 mol/L hydrochloric acid circulating about 15min, after thoroughly washed with water, high purity aqueous sodium hypophosphite aqueous solution (15 g/60 ml H2O) flows through it, the resin column is first washed with 50 ml, then with 25 rnl distilled water. The effluent acid and washing is combined, it is concentrated by evaporation in water bath. The concentrated acid is placed in high vacuum with P205 dryer for dehydration, cooling and crystallization, filtration, recrystallization, to obtain hypophosphorous acid product. | | Production method | Ion exchange resin method: put about 70 g water-soluble cation exchange resins to fill into a glass tube. Circulate with 5 mol/L hydrochloric acid for about 15 min and wash sufficiently with water. Have a high aqueous sodium hypophosphite solution (15 g/60 ml H2O) to flow through the resin column, followed by being washed first with 50 ml water, and then rinsing with 25 rnl distilled water. The effluent acid and the washings were combined and concentrated by evaporation on a water bath. The concentrated acid is send to the highly vacuum, P205 dryer for dehydration, followed by cooling crystallization, filtration and recrystallization to obtain the finished product of hypophosphorous acid. | | Description | This acid has the general formula ofH4P2O6 and differs
from the other oxy-phosphorous acids. It has many peculiarities.
It is formed along with phosphorous and phosphoric
acids, when phosphorus is oxidized by moist air.
If white phosphorus is exposed to air, and sodium acetate
is addedto the liquidwhich forms, the somewhat insoluble
sodium hypophosphate,Na2H2P2O6·6H2Oseparates. The
sodium hypophosphate monohydrate, however, is very
soluble and deliquescent at ~98.7 g/100 ml. | | Description | Hypophosphorous acid is a powerful reducing agent
with a molecular formula of H3PO2. Inorganic chemists
refer to the free acid by this name although its IUPAC
name is dihydridohydroxidooxidophosphorus, or the
acceptable name of phosphinic acid. It is a colorless
low-melting compound, which is soluble in water,
dioxane, and alcohols. The formula for hypophosphorous
acid is generally written H3PO2, but a more descriptive presentation is HOP(O)H2 which highlights
its monoprotic character. Salts derived from this acid
are called phosphinates (hypophosphites). | | Chemical Properties | colourless liquid | | Physical properties | Colorless deliquescent crystals or oily liquid; sour odor; density 1.493 g/cm3;melts at 26.5°C; boils at 130°C; very soluble in water, alcohol and ether; den-sity of a 50% aqueous solution is 1.13 g/mL. | | Uses | Hypophosphorous acid is primarily used for electroless nickel plating. It is involved in the reduction of arenediazonium salts. It acts as an additive in Fischer esterification reactions. Also, it serves as a neutralizing agent, antioxidant, catalyst in polymerization and poly condensation, and wetting agent. Further, it is used in the formulation of pharmaceuticals, discoloration of polymers, water treatment and retrieval of precious or non-ferrous metals. In addition to this, it is used as bleaching agents for plastics, synthetic fibers, decolorizing agent and for color stabilization during the manufacture of chemicals and several plastics. | | Production Methods | Hypophosphorous acid is formed by reaction of barium hypophosphite and sulfuric acid, and filtering off barium sulfate. By evaporation of the solution in vacuum at 80 °C, and then cooling to 0°C, hypophosphorous acid crystallizes. | | Definition | A white crystalline solid. It is a monobasic acid forming the anion H2PO2 – in water. The sodium salt, and hence the acid, can be prepared by heating yellow phosphorus with sodium hydroxide solution. The free acid and its salts are powerful reducing agents. | | Definition | ChEBI: A phosphorus oxoacid that consists of a single pentavalent phosphorus covalently bound via single bonds to two hydrogens and a hydroxy group and via a double bond to an oxygen. The parent of the class of phosphinic acids. | | Preparation | Hypophosphorous acid may be prepared by various methods:
1. Boiling white phosphorus with calcium hydroxide:
P4 + 4Ca(OH)2 + 8H2O → 4Ca(H2PO2)2 + 4H2
The calcium salt is soluble in water. Treatment with sulfuric acid yields thehypophosphorous acid:
(H2PO2)2Ca + H2SO4 → 2H3PO2 + CaSO4
The product mixture is filtered to remove insoluble CaSO4. The aqueous solu-tion of hypophosphorous acid is concentrated under reduced pressure.Concentrated baryta water may be used instead of calcium hydroxide.2. By treating sodium hypophosphite, NaH2PO2with an ion-exchange resin.The sodium salt may be produced by boiling white phosphorus with a solutionof sodium hydroxide, a reaction similar to (1) above.
PH3 + 2I2 + 2H2O → H3PO2 + 4HI
The above method may be considered safer than that involving heating whitephosphorus with an alkali.
Hypophosphorous acid must be stored below 50°C. It is sold commerciallyas an aqueous solution at various concentrations. | | Reactions | Hypophosphorous acid is miscible with water in all proportions and a commercial strength is 30% H3PO2. Hypophosphites are used in medicine. Hypophosphorous acid is a powerful reducing agent, e.g., with copper sulfate forms cuprous hydride Cu2H2, brown precipitate, which evolves hydrogen gas and leaves copper on warming; with silver nitrate yields finely divided silver; with sulfurous acid yields sulfur and some hydrogen sulfide; with sulfuric acid yields sulfurous acid, which reacts as above; forms manganous immediately with permanganate. | | General Description | Hypophosphorous acid appears as colorless oily liquid or deliquescent crystals with a sour odor. Density 1.439 g / cm3. Melting point 26.5°C. Inhalation of vapors irritates or burns the respiratory tract. Liquid and vapors may irritate or burn eyes and skin. | | Air & Water Reactions | Deliquescent. Water soluble. | | Reactivity Profile | HYPOPHOSPHOROUS ACID decomposes when heated into phosphoric acid and spontaneously flammable phosphine. Is oxidized by sulfuric acid with release of sulfur dioxide and sulfur. Reacts explosively with mercury(II) oxide [Mellor, 1940, Vol. 4, 778]. Reacts violently with mercury(II) nitrate [Mellor, 1940, Vol. 4, 993]. Neutralizes bases in exothermic reactions. | | Hazard | Fire and explosion risk in contact with oxidizing agents. | | Health Hazard | TOXIC; inhalation, ingestion or skin contact with material may cause severe injury or death. Contact with molten substance may cause severe burns to skin and eyes. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution. | | Fire Hazard | Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Some are oxidizers and may ignite combustibles (wood, paper, oil, clothing, etc.). Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated. | | Purification Methods | Phosphorous acid is a common contaminant of commercial 50% hypophosphorous acid. Jenkins and Jones [J Am Chem Soc 74 1353 1952] purified this material by evaporating about 600mL in a 1L flask at 40o, under reduced pressure (in N2), to a volume of about 300mL. After the solution was cooled, it was transferred to a wide-mouthed Erlenmeyer flask which was stoppered and left in a Dry-ice/acetone bath for several hours to freeze (if necessary, with scratching of the wall). When the flask was then left at ca 5o for 12hours, about 30-40% of it liquefied, and was again filtered. This process was repeated, then the solid was stored over Mg(ClO4)2 in a vacuum desiccator in the cold. Subsequent crystallisations from n-butanol by dissolving it at room temperature and then cooling in an ice-salt bath at -20o did not appear to purify it further. The free acid forms deliquescent crystals m 26.5o and is soluble in H2O and EtOH. The NaH2PO2 salt can be purified through an anion exchange resin [Klement Z Anorg Allgem Chem 260 267 1949.] |
| | Hypophosphorous acid Preparation Products And Raw materials |
|