- Zinc chromate
-

- $10.00 / 1KG
-
2023-04-07
- CAS:13530-65-9
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 10 mt
- Zinc chroMate
-

- $10.00 / 1KG
-
2020-06-05
- CAS:13530-65-9
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 20 tons
- Zinc chromate
-

- $1.00 / 1KG
-
2020-01-10
- CAS:13530-65-9
- Min. Order: 1g
- Purity: 98%
- Supply Ability: 200kgs
|
| Product Name: | Zinc chromate | | Synonyms: | basiczincchromate;buttercupyellow;c.i.77955;chromicacid(h2cro4),zincsalt(1:1);chromicacid,zincsalt;Chromicacid,zincsalt(1:1);chromiumzincoxide(zrcro4);ci77955 | | CAS: | 13530-65-9 | | MF: | CrH2O4.Zn | | MW: | 181.38 | | EINECS: | 236-878-9 | | Product Categories: | Inorganics | | Mol File: | 13530-65-9.mol | 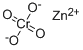 |
| | Zinc chromate Chemical Properties |
| density | 3,4 g/cm3 | | solubility | soluble in acid solutions; insoluble in acetone | | Melting point | 316 °C | | color | yellow prisms | | Water Solubility | Insoluble in water. | | Exposure limits | ACGIH: TWA 0.0002 mg/m3; STEL 0.0005 mg/m3 (Skin)
OSHA: Ceiling 0.1 mg/m3
NIOSH: IDLH 15 mg/m3; TWA 0.0002 mg/m3 | | CAS DataBase Reference | 13530-65-9(CAS DataBase Reference) | | EPA Substance Registry System | Zinc chromate (13530-65-9) |
| Provider | Language |
|
ALFA
| English |
| | Zinc chromate Usage And Synthesis |
| Description | Zinc Chromate is a zinc salt of chromate. As a corrosion inhibitor, it is used industrially in chromate conversion coatings, which was developed by the Ford Motor Company in the 1920s. Its major application is used as a coating over iron or aluminum materials in industrial painting with corrosion resistance. It is also used in spray paints, artists'paints, pigments in varnishes, metal surface treatment and in making linoleum. Its putty form can be used as sealant in addition to two O-rings between sections of the failed solid rocket booster on Space Shuttle Challenger. However, it was concerned that it is a potential carcinogen, which can induce chromosome instability and DNA double-strand breaks.

| | Uses | Zinc chromate is also called zinc yellow, this pigment is identified as Pigment Yellow 36, as opposed to the lithopone version incorpo- rating barium sulfate which is Pigment Yellow 36:1. It is a bright, green shade of yellow made by the precipitation of hydrated zinc potassium chromate from the reaction of so- dium bichromate with zinc oxide and potassium chloride. The lithopone version is merely a co-precipitate of zinc chromate onto barium sulfate to give an extended pigment that carries the Colour Index name of Pigment Yellow 36:1.
Used primarily in corrosion-inhibiting coatings, its poor tinctorial strength and poor resistance to acid and alkali severely limits this pigment's use elsewhere.
| | Referrence |
- Tencer, Michal (30 September 2006). "Electrical conductivity of chromate conversion coating on electrodeposited zinc". Applied Surface Science. 252 (23): 8229–8234.
- J.A. Hickman (1997). Polymeric Seals and Sealing Technology. iSmithers Rapra Publishing. p. 25. ISBN 978-1-85957-096-8. Retrieved 24 March 2011.
- Xie, Hong, et al. "Zinc Chromate Induces Chromosome Instability and DNA Double Strand Breaks in Human Lung Cells." Toxicology & Applied Pharmacology 234.3(2009):293-9.
| | Chemical Properties | Zinc chromate is a yellow crystalline powder. | | Uses | Zinc yellow has the composition
4ZnCrO4 ? K2O ? H2O. Two other yellow chromate pigments are strontium
chromate and barium chromate, both used as corrosion inhibitors. | | Uses | Zinc chromate is used in pigments, rubber, linoleum manufacturing, metal surface treatment, electroplating intermediates, research and development. It finds its application as an useful metal for preventing corrosion when applied to other metals. | | General Description | Odorless yellow solid. Sinks in water. | | Reactivity Profile | Oxidizing agents, such as ZINC CHROMATE, can react with reducing agents to generate heat and products that may be gaseous (causing pressurization of closed containers). The products may themselves be capable of further reactions (such as combustion in the air). The chemical reduction of materials in this group can be rapid or even explosive, but often requires initiation (heat, spark, catalyst, addition of a solvent). Explosive mixtures of inorganic oxidizing agents with reducing agents often persist unchanged for long periods if initiation is prevented. Such systems are typically mixtures of solids, but may involve any combination of physical states. Some inorganic oxidizing agents are salts of metals that are soluble in water; dissolution dilutes but does not nullify the oxidizing power of such materials. Organic compounds, in general, have some reducing power and can in principle react with compounds in this class. Actual reactivity varies greatly with the identity of the organic compound. Inorganic oxidizing agents can react violently with active metals, cyanides, esters, and thiocyanates. | | Health Hazard | Inhalation of dust causes irritation of nose and throat. Ingestion can cause irritation or corrosion of the alimentary tract, circulatory collapse, and toxic nephritis. Contact with eyes or skin causes irritation. | | Safety Profile | Confirmed human
carcinogen producing lung tumors. A
poison via intravenous route. Human
mutation data reported. See also
CHROMIUM COMPOUNDS and ZINC
COMPOUNDS. | | Potential Exposure | Zinc chromate is used as an anticorrosion
pigment in primers and as a coloring agent;
as a pigment in surface coatings and linoleum; to impart
corrosion resistance to epoxy laminates. | | Shipping | UN3288 Toxic solids, inorganic, n.o.s., Hazard
Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name
Required. UN3077 Environmentally hazardous substances,
solid, n.o.s., Hazard class: 9; Labels: 9-Miscellaneous hazardous
material, Technical Name Required. | | Incompatibilities | An oxidizer; reacts with reducing agents;
combustibles, organic materials. |
| | Zinc chromate Preparation Products And Raw materials |
|