|
ChemicalBook Optimization Suppliers |
|
| | 4-ヒドロキシベンズアルデヒド Usage And Synthesis |
| 外観 | 白色~うすい黄褐色、結晶性粉末~粉末 | | 溶解性 | 熱水, エタノール, エーテルに可溶。水にやや溶けにくく、エタノールに溶ける。 | | 用途 | 分析試薬
| | 用途 | 有機合成原料、香料材料。 | | 化学的特性 | 4-Hydroxybenzaldehyde is a yellow to light brown crystalline powder that has a very faint, sweet-woody-balsamic odor and a sweet taste with little or no other flavor impression. The odor is also reported as vanillic/nutty. | | 天然物の起源 | It is found as a volatile in several food products, including cherries, grapes, papayas, tomatoes, cheese, beer,
rum, brandy, wine, tea and peanuts. Occurs in the form of esters in several plants, notably in wintergreen leaves and the bark of sweet
birch. | | 使用 | 4-Hydroxybenzaldehyde maintains bactericidal activity when tested against certain bacteria strains. It also displays antioxidant potential when analyzed through assay. It is widely used starting material for polymers and pharmaceuticals. | | 主な応用 | p-Hydroxybenzaldehyde is the important intermediates of pharmaceutical industry and spices. In foreign , it's also used for synthesis of bromoxynil and chloroxynil which are kind of herbicides, and also used in the manufacture of bactericide, photographic emulsifier, nickel plating luster agent, liquid crystal, etc; In the pharmaceutical field, it can be used for synthesis of amoxicillin, antibacterial synergistic agent named TMP, 3,4,5-Trimethoxybenzaldehyde,Artificial gastrodia elata, farrerol, esmololhydrochloride; In the spicery field, it can be used for synthesis of spicery,for example: vanillin, ethyl vanillin, piperonal, springaldehyde, p-anisaldehyde, raspberry ketone natural,etc. | | 製造方法 | p-Hydroxybenzaldehyde is prepared by heating sodium phenolate with carbon dioxide under pressure. | | 定義 | ChEBI: 4-hydroxybenzaldehyde is a hydroxybenzaldehyde that is benzaldehyde substituted with a hydroxy group at position C-4. It has a role as a plant metabolite, a mouse metabolite and an EC 1.14.17.1 (dopamine beta-monooxygenase) inhibitor. | | Synthesis Reference(s) | Synthetic Communications, 9, p. 407, 1979 DOI: 10.1080/00397917908064169 | | 一般的な説明 | 4-hydroxybenzaldehyde occurs naturally in vanilla beans and is one of the keys contributors to the vanilla flavor. | | 合成 | 2,3-Dichloro-5,6-dicyano-p-benzoquinone (DDQ 908 mg, 4 mmol) was added to a solution of 4-hydroxybenzyl alcohol (496 mg, 4 mmol) in dioxane (24 mL). The reaction mixture immediately turned deep green (exothermic reaction), and DDQH2 started precipitating within 1 min. Thin layer chromatography (TLC) analysis indicated consumption of starting material after 15 min. The solvent was removed from the yellow reaction mixture in vacuo. Treatment of the residue with CH2Cl2 left DDQH2 undissolved (quantitatively). Filtration followed by evaporation of CH2Cl2 gave 4-hydroxybenzaldehyde (74% yield) which was recrystallized from water.
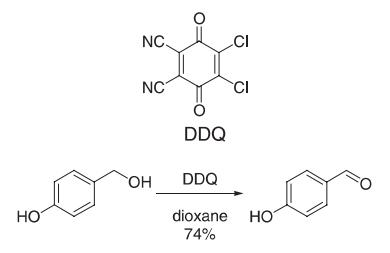
Reference: Becker, H.-D.; Bjork, A.; Alder, E. J. Org. Chem. 1980, 45, 1596?1600. | | target | GABA Receptor | | 純化方法 | Crystallise it from water (containing some H2SO4). Dry it over P2O5 under vacuum. [Beilstein 8 H 64, 8 IV 251.] | | 参考文献 | [1] HANS DIETER BECKER Erich A Anders Bjoerk. Quinone dehydrogenation. Oxidation of benzylic alcohols with 2,3-dichloro-5,6-dicyanobenzoquinone[J]. The Journal of Organic Chemistry, 1980, 45 9: 1596-1600. DOI:10.1021/jo01297a010.
[2] CHAN WOO KANG. 4-Hydroxybenzaldehyde accelerates acute wound healing through activation of focal adhesion signalling in keratinocytes.[J]. Scientific Reports, 2017: 14192. DOI:10.1038/s41598-017-14368-y.
[3] M. EDDOUKS. Insulin Resistance as a Target of Some Plant-Derived Phytocompounds[J]. Studies in natural products chemistry, 1900, 26 1: 351-373. DOI:10.1016/B978-0-444-63430-6.00011-4.
[4] DOUGLASS F. TABER. Vanillin Synthesis from 4-Hydroxybenzaldehyde[J]. Journal of Chemical Education, 2007, 84 7: 1158. DOI:10.1021/ed084p1158.
[5] MAKOTO KOMIYAMA Hidefumi H. Selective synthesis of 4-hydroxybenzaldehyde with cyclodextrin as catalyst[J]. Macromolecular Rapid Communications, 1981, 2 12: 715-717. DOI:10.1002/marc.1981.030021202.
[6] HIROAKI MATSUDA . Mutagenicity of ozonation and chlorination products from p-hydroxybenzaldehyde[J]. Science of the Total Environment, 1991, 103 2: Pages 141-149. DOI:10.1016/0048-9697(91)90140-A. |
|