- 8-Methoxypsoralen
-

- $0.00 / 1kg
-
2023-10-09
- CAS:298-81-7
- Min. Order: 1kg
- Purity: 0.99
- Supply Ability: 20tons
- 8-Methoxypsoralen
-

- $0.00 / 25KG
-
2023-09-01
- CAS:298-81-7
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 20tons
- 8-Methoxypsoralen
-
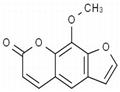
- $0.00 / 20mg
-
2023-02-24
- CAS:298-81-7
- Min. Order: 5mg
- Purity: ≥98%(HPLC)
- Supply Ability: 10 g
|
| Product Name: | 8-Methoxypsoralen | | Synonyms: | 8-Methoxy-2',3',6,7-furocoumarin;8-methoxy-2’,3’,6,7-furocoumarin;8-Methoxy-4',5',6,7-furocoumarin;8-Methoxy-4',5':6,7-furocoumarin;8-methoxy-4’,5’,6,7-furocoumarin;8-methoxy-4’,5’:6,7-furocoumarin;8-Methoxy-6,7-furanocoumarin;8-Methoxyfuranocoumarin | | CAS: | 298-81-7 | | MF: | C12H8O4 | | MW: | 216.19 | | EINECS: | 206-066-9 | | Product Categories: | Cytochrome P450 isozyme;This product is manufactured in GMP workshop.;Aromatics;Heterocycles;Intermediates & Fine Chemicals;Pharmaceuticals;Other APIs;Inhibitors;chemical reagent;pharmaceutical intermediate;phytochemical;reference standards from Chinese medicinal herbs (TCM).;standardized herbal extract;OXSORALENE;API;john's | | Mol File: | 298-81-7.mol | 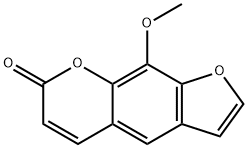 |
| | 8-Methoxypsoralen Chemical Properties |
| Melting point | 148-150 °C(lit.) | | Boiling point | 276.6°C (rough estimate) | | density | 1.1344 (rough estimate) | | refractive index | 1.4270 (estimate) | | storage temp. | Sealed in dry,Room Temperature | | solubility | H2O: slightly soluble | | form | Needle-Like Crystals or Powder | | color | white to yellow | | Water Solubility | PRACTICALLY INSOLUBLE | | Merck | 14,5988 | | BRN | 196453 | | LogP | 1.523 (est) | | CAS DataBase Reference | 298-81-7(CAS DataBase Reference) | | NIST Chemistry Reference | Methoxsalen(298-81-7) | | IARC | 1 (Vol. 24, Sup 7, 100A) 2012 | | EPA Substance Registry System | Methoxsalen (298-81-7) |
| | 8-Methoxypsoralen Usage And Synthesis |
| Description | 8-methoxypsoralen (also known as methoxsalen) is a naturally occurring furocoumarin compound existing in several species of plants such as Psoralea corylifolia. It is a photoactive substance that can form DNA adducts upon UVA irradiation. It is a drug used for the treatment of psoriasis, eczema, vitiligo, and some kinds of cutaneous lymphomas associated with the exposure of skin to the UVA or light from lamps or sunlight. Its mechanism of action is through inhibiting the synthesis of the deoxyribonucleic acid (DNA). After the activation, it can preferentially bind to the guanine and cytosine molecules of DNA, further leading to the cross-linking of DNA, thus inhibiting the DNA synthesis. It can further inhibit the RNA and protein synthesis at high concentration. It is extracted from Ammi majus.
| | References | https://www.drugbank.ca/drugs/DB00553
https://en.wikipedia.org/wiki/Methoxsalen
| | Description | 8-Methoxypsoralen (8-MOP) and other psoralens are naturally
found in plants, including common fruit and vegetable crops. | | Chemical Properties | White to cream-colored, crystalline
solid; odorless. Slightly soluble in alcohol; practically insoluble in water. Combustible. | | Originator | Oxsoracen,Eider,US,1955 | | Uses | A potent suicide inhibitor of cytochrome P-450. | | Uses | For the treatment of psoriasis and vitiligo | | Uses | antipsoriatic, pigmentation agent | | Uses | Naturally occurring analog of psoralen. Use in treatment of psoriasis and mycosis fungoides | | Uses | Naturally occurring analog of Psoralen (P839800). Use in treatment of psoriasis and mycosis fungoides. | | Uses | 8-Methoxypsoralen, is used in Photochemotherapy (methoxsalen with long wave ultraviolet radiation) is indicated for the repigmentation of idiopathic vitiligo. It is also used in Photopheresis (methoxsalen with long wave ultraviolet radiation of white blood cells) is indicated for use with the UVAR* System in the palliative treatment of the skin manifestations of cutaneous T-cell lymphoma. | | Indications | Methoxsalen has effects similar to those of trioxsalen. Methoxsalen is superior
to trioxsalen in producing erythema and tanning and is the drug used in PUVA
therapy. Methoxsalen is also available as a 1% lotion. | | Definition | ChEBI: A member of the class of psoralens that is 7H-furo[3,2-g]chromen-7-one in which the 9 position is substituted by a methoxy group. It is a constituent of the fruits of Ammi majus. Like other psoralens, trioxsalen
causes photosensitization of the skin. It is administered topically or orally in conjunction with UV-A for phototherapy treatment of vitiligo and severe psoriasis. | | Manufacturing Process | It has been found that the compound 8-geranoxy psoralen is present in citrus
oils, particularly lemon and lime oils. This compound can be isolated from the
oil by a process which involves primarily absorption on an adsorbent material
followed by elution with a suitable solvent.
(A) Cleavage of 8-Geranoxypsoralen: 275 mg of 8-geranoxypsoralen was
dissolved with mechanical stirring in 4 ml glacial acetic acid. After 10 minutes,one drop of concentrated sulfuric acid was added to the solution. In 4 minutes
thereafter a light tan precipitate began to form. Stirring was continued for 35
minutes and the reaction mixture was refrigerated for one hour and 20
minutes. The precipitate was then removed by suction filtration and washed
on the filter with glacial acetic acid followed by ice-cold ethyl ether. The
product, 8-hydroxypsoralen, weighed 115 mg, that is, 74% of theory.
(B) Methylation of 8-Hydroxypsoralen: 115 mg of 8-hydroxypsoralen was
dissolved in 10 ml absolute methanol, an excess of diazomethane dissolved in
ether was added and the mixture allowed to stand at room temperature with
occasional stirring for 3 hours. The next day the reaction mixture was reduced
in volume to 3 ml by evaporation on the steam bath and the concentrate was
held in a refrigerator overnight. The next day, fine needles (80 mg) of 8-
methoxypsoralen were filtered from the solution. The compound had a MP of
145 to 146°C and was obtained in a yield of 65% of theory.
There is also a wholly synthetic route to Methoxsalen as outlined by Kleeman
and Engel. | | Brand name | 8-Mop (Valeant); Oxsoralen (Valeant); Uvadex (Therakos). | | Therapeutic Function | Dermal pigmentation enhancer | | General Description | Odorless white to cream-colored crystalline solid. Bitter taste followed by tingling sensation. | | Air & Water Reactions | Sensitive to light and air. Insoluble in water. | | Reactivity Profile | 8-Methoxypsoralen is incompatible with strong oxidizing agents. | | Fire Hazard | Flash point data for 8-Methoxypsoralen are not available; however, 8-Methoxypsoralen is probably combustible. | | Contact allergens | This fur(an)ocoumarin is an phototoxic compound
that causes phototoxic dermatitis. Many plants of the
Apiaceae–Umbelliferae and most of the Rutaceae family
contain 5-methoxypsoralen and 8-methoxypsoralen.
Their spectra is in the UVA range (300–360 nm). It is
used in combination with UVA to treat various skin
disorders such as psoriasis. | | Safety Profile | Confirmed carcinogen.
Poison by intraperitoneal route. Moderately
toxic by ingestion and subcutaneous routes.
Human mutation data reported. When
heated to decomposition it emits acrid
smoke and irritating fumes. A drug used to
treat slun diseases. | | Environmental Fate | The industrial use of 8-MOP results in its release into the
environment through multiple pathways, and its existence as
a natural substance in plants further expands exposure to the
environment. Airborne 8-MOP will exist in the vapor and
particulate phases, and will be degraded in air by reaction with
photochemically produced hydroxyl radicals, with an estimated
half-life of approximately 1.2 h, it may also be subject to
direct photolysis by sunlight. Particulate 8-MOP will be
removed from the atmosphere by wet or dry deposition. If
released into the soil, it is expected to have high mobility and is
not expected to volatilize. 8-MOP does not biodegrade. In
aqueous environments, 8-MOP is not expected to hydrolyze, it
will, however, adsorb to suspended solids and sediment. Due
to 8-MOP’Ks resistance to degradation by many routes, it is
expected to remain in the environment for a prolonged period,
and as such will also be subject to long-range transport. 8-MOP
has an estimated bioconcentration factor (BCF) of 9, meaning
that bioconcentration and bioaccumulation are low in aquatic
organisms. | | Purification Methods | Purify xanthotoxin by recrystallisation from *C6H6/pet ether (b 60-80o) to give silky needles, or from EtOH/Et2O to give rhombic prisms or from hot H2O to give needles. It is soluble in aqueous alkali due to ring opening of the cyclic lactone but recyclises upon acidification. It has UV max in EtOH at 219, 249 and 300nm (log � 4.32, 4.35 and 4.06) and 1H NMR in CDCl3 with � at 7.76 (d, 1H, J 10 Hz), 7.71 (d, 1H, J 2.5 Hz), 7.38 (s, 1H), 6.84 (d, 1H, J 2.5 Hz), 6.39 (d, 1H, J 10 Hz) and 4.28 (s, 3H)ppm. [Nore & Honkanen J Heterocycl Chem 17 985 1980.] It is a DNA intercalator, is used in the treatment of dermal diseases, and is a human carcinogen [Tessman et al. Biochemistry 24 1669 1985.] [Beilstein 19 I 711, 19/6 V 15.] | | Toxicity evaluation | The toxic effects of psoralens almost never occur without
exposure to UV light. These are photosensitizing materials that
exert their primary effect on the skin. 8-MOP, when activated
by long-wavelength UV light in the range of 320–400 nm, is
strongly erythemogenic, melanogenic, and cytotoxic in the
epidermis. The mechanisms of action of 8-MOP in inducing
repigmentation of vitiliginous skin have not been established.
Repigmentation depends on the presence of functioning
melanocytes and UV light. 8-MOP may activate the functional
and dihydroxyphenylalanine-positive melanocytes present in
vitiliginous skin. An increase in the activity of tyrosinase, the
enzyme that catalyzes the conversion of tyrosine to dihydroxyphenylalanine
(a precursor of melanin), has been shown in melanin-producing cells exposed in vitro to trioxsalen and UVA
light. In addition, binding of photoactivated psoralens (in
triplet states) to pyrimidine bases of nucleic acids, with
subsequent inhibitions of DNA synthesis, cell division, and
epidermal turnover, has been demonstrated. Following photoactivation,
8-MOP forms covalent bonds with DNA to
produce monofunctional (addition to a single strand of DNA)
and bifunctional adducts (cross-linking to both strands of
DNA). Reactions with other proteins also occur. Psoralens may
also increase melanin formation by producing an inflammatory
reaction in the skin. Other mechanisms of increased
pigmentation may include an increase in the number of functional
melanocytes (and possibly activation of dormant
melanocytes); enhancement of melanin granule synthesis;
stimulation of the movement of melanocytes up hair follicles
resulting in melanocytic repopulation of the epidermis; and/or
hypertrophy of melanocytes and increased arborization of their
dendrites. Since psoriasis is a hyperproliferative disorder and
other agents effective in the treatment of psoriasis are known to
inhibit DNA synthesis, the therapeutic effect of 8-MOP in the
treatment of psoriasis probably involves binding to DNA and
inhibition of DNA synthesis resulting in decreased cell proliferation;
other vascular, leukocyte, or cell regulatory mechanisms
may be involved. It has been suggested that at low drug
load, 8-MOP binds to DNA as an intercalator, whereas at higher
ratios of 8-MOP to DNA, it binds to the outside of DNA,
probably in the minor groove and causes some compaction in
DNA. Protective eyewear is used to prevent irreversible binding
of 8-MOP to proteins and DNA components of the lens. The
central hypothesis for the reproductive toxicity of 8-MOP is
that it produces reproductive effects by disrupting the hypothalamus–
pituitary axis, and the alternative hypothesis is that
this compound targets gonadal function, resulting in alteration
of pregnancy outcome. |
| | 8-Methoxypsoralen Preparation Products And Raw materials |
|