| | N,N-Diethyl-4-nitrosoaniline Basic information |
| Product Name: | N,N-Diethyl-4-nitrosoaniline | | Synonyms: | p-Nitroso-N,N-diethylaniline;n,n-diethyl-p-nitroso-anilin;N,N-DIETHYL-P-NITROSOANILINE;N,N-DIETHYL-4-NITROSOANILINE;P-NITROSODIETHYLANILINE;N N-DIETHYL-4-NITROSOANILINE 97%;N,N-Diethyl-4-nitrosoaniline,98%;N,N-Diethyl-4-nitrosoaniline | | CAS: | 120-22-9 | | MF: | C10H14N2O | | MW: | 178.23 | | EINECS: | 204-379-5 | | Product Categories: | | | Mol File: | 120-22-9.mol | 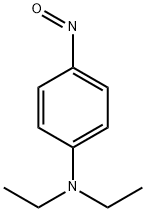 |
| | N,N-Diethyl-4-nitrosoaniline Chemical Properties |
| Hazard Codes | T-F,T | | Risk Statements | 33-23/24/25-11 | | Safety Statements | 45-36/37/39-22-16 | | RIDADR | 2926 | | RTECS | BX3610000 | | HazardClass | 4.1 | | PackingGroup | II | | HS Code | 2921420090 | | Toxicity | mmo-sat 3 mmol/L XENOBH 16,587,86 |
| | N,N-Diethyl-4-nitrosoaniline Usage And Synthesis |
| Chemical Properties | green crystalline powder | | Uses | N,N-Diethyl-4-nitrosobenzenamine reacts with rubber to form a compound that acts as efficient antioxidants. | | General Description | Green solid. Insoluble in water. | | Air & Water Reactions | Insoluble in water. | | Reactivity Profile | A nitrated amine. Amines are combustible. The combustion of amines yields noxious NOx. Amines are chemical bases. They neutralize acids to form salts plus water. These acid-base reactions are exothermic. The amount of heat that is evolved per mole of amine in a neutralization is largely independent of the strength of the amine as a base. Amines may be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen is generated by amines in combination with strong reducing agents, such as hydrides. Aromatic nitro compounds range from slight to strong oxidizing agents. If mixed with reducing agents, including hydrides, sulfides and nitrides, they may begin a vigorous reaction that culminates in a detonation. The aromatic nitro compounds may explode in the presence of a base such as sodium hydroxide or potassium hydroxide even in the presence of water or organic solvents. The explosive tendencies of aromatic nitro compounds are increased by the presence of multiple nitro groups. | | Safety Profile | Mutation data reported. When heated to decomposition it emits toxic vapors of NOx. |
| | N,N-Diethyl-4-nitrosoaniline Preparation Products And Raw materials |
|