|
|
| | 2,4-Dichlorobenzoyl peroxide Basic information |
| Product Name: | 2,4-Dichlorobenzoyl peroxide | | Synonyms: | tc2(peroxide);Di-2,4-dichlorobenzoyl peroxide;2,4-DICHLOROBENZOYL PEROXIDE;Bis-(2,4-dichlorbenzoyl)peroxid;2,4-DICHLOROBENZOYL PEROXIDE: 50% IN POLYDIMETHYLSILOXANE;2 4-DICHLOROBENZOYL PEROXIDE 50% PASTE;2,4-dichlorobenzoic peroxyanhydride;o,o',p,p'-Tetrachlorodibenzoyl peroxide | | CAS: | 133-14-2 | | MF: | C14H6Cl4O4 | | MW: | 380.01 | | EINECS: | 205-094-9 | | Product Categories: | Pharmaceutical intermediates | | Mol File: | 133-14-2.mol | 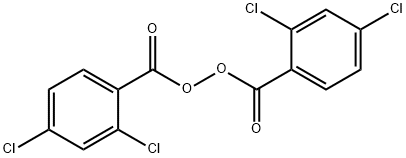 |
| | 2,4-Dichlorobenzoyl peroxide Chemical Properties |
| Melting point | 55°C (dec.) | | Boiling point | 495.27°C (rough estimate) | | density | 1,26 g/cm3 | | vapor pressure | 0.009Pa at 25℃ | | refractive index | 1.5282 (estimate) | | Specific Gravity | 1.26 | | Water Solubility | 29.93μg/L at 25℃ | | Hydrolytic Sensitivity | 4: no reaction with water under neutral conditions | | LogP | 6 at 20℃ | | CAS DataBase Reference | 133-14-2(CAS DataBase Reference) | | EPA Substance Registry System | Peroxide, bis(2,4-dichlorobenzoyl) (133-14-2) |
| Safety Statements | 15-17 | | RIDADR | 3106 | | TSCA | Yes | | Toxicity | LD50 ipr-mus: 225 mg/kg IPSTB3 3,93,76 |
| | 2,4-Dichlorobenzoyl peroxide Usage And Synthesis |
| General Description | 3,5-dichlorocatechol (3,5-DCK), 2,4-dichlorophenol (2,4-DCP), and 3,5-dichlorophenol (3,5-DCP) are metabolites of 2,4-Dichlorobenzoyl peroxide in the human body. They are excreted in the urine[1].
| | Chemical Properties | DCBP is a white to yellowish viscous paste, diacyl peroxide,
cross-linking agent of silicone rubber with 150–400°C hot air
without pressure. Fifty percent in paste is available for use.
No additional information was found. | | Uses | Bis(2,4-dichlorobenzoyl)peroxide (2,4-DCBP), also known as 2,4-Dichlorobenzoyl peroxide, is commonly used as an initiator or cross-linking agent for silicone rubber production. During hot curing, 2,4-DCBP decomposes into 2,4-dichlorobenzoic acid, 1,3-dichlorobenzene, and the polychlorinated biphenyl (PCB) congeners PCB-47, PCB-51, and PCB-68. Due to the high persistence of PCBs and the poorly defined toxicological properties of PCB 47 and PCB 68, a replacement of 2,4-DCBP as initiator in silicone rubber production should be considered[1].
| | Reactivity Profile | May explode from heat, shock, friction or contamination. May ignite combustibles (wood, paper, oil, clothing, etc.). May be ignited by heat, sparks or flames. Peroxides are good oxidizing agents. Organic compounds can ignite on contact with concentrated peroxides. Strongly reduced material such as sulfides, nitrides, and hydrides may react explosively with peroxides. There are few chemical classes that do not at least produce heat when mixed with peroxides. Many produce explosions or generate gases (toxic and nontoxic). Generally, dilute solutions of peroxides (<70%) are safe, but the presence of a catalyst (often a transition metal such as cobalt, iron, manganese, nickel, or vanadium) as an impurity may even then cause rapid decomposition, a buildup of heat, and even an explosion. Solutions of peroxides often become explosive when evaporated to dryness or near-dryness. | | Hazard | 2,4-Dichlorobenzoyl peroxide may damage fertility or the unborn child, if heated may cause a fire and may cause an allergic skin reaction. | | Safety Profile | Poison by intraperitoneal route.Explosion Hazard: Pure compound is extremely shocksensitive and decomposes rapidly @ 80°. When heated todecomposition it emits toxic fumes of Cl-. | | References | [1] Schettgen T, et al. Decomposition Products of the Initiator Bis(2,4-dichlorobenzoyl)peroxide in the Silicone Industry: Human Biomonitoring in Plasma and Urine of Workers. Environmental Science & Technology, 2022; 56: 8518–8527.
|
| | 2,4-Dichlorobenzoyl peroxide Preparation Products And Raw materials |
|