|
ChemicalBook Optimization Suppliers |
|
| 化学名: | 1,2-ジクロロエタン | | 英語化学名: | 1,2-Dichloroethane | | 别名: | 1,2-Bichloroethane;1,2-Dichlor-aethan;1,2-dichloro-ethan;1,2-dichloroethane (ethylene dichloride);1,2-dichloroethane,anhydrous;1,2-Dichlorαthan;1,2-Dichlorαthan(Hochtemperaturform);1,2-Dicloroetano | | CAS番号: | 107-06-2 | | 分子式: | C2H4Cl2 | | 分子量: | 98.96 | | EINECS: | 203-458-1 | | カテゴリ情報: | Anhydrous Solvents;Solvent by Application;Solvents;ACS and Reagent Grade Solvents;ACS Grade;ACS Grade Solvents;Amber Glass Bottles;Carbon Steel Cans with NPT Threads;Semi-Bulk Solvents;Solvent Bottles;Solvent Packaging Options;Anhydrous;Products;Returnable Containers;Sure/Seal Bottles;Analytical Reagents;Analytical Reagents for General Use;Analytical/Chromatography;C-D;Histological Solvents;Puriss p.a.;Alpha Sort;D;DAlphabetic;DIA - DIC;Volatiles/ Semivolatiles;alpha,omega-Bifunctional Alkanes;alpha,omega-Dichloroalkanes;Analytical Chemistry;Monofunctional & alpha,omega-Bifunctional Alkanes;Solvents for HPLC & Spectrophotometry;Solvents for Spectrophotometry;refrigerants;Organics | | Mol File: | 107-06-2.mol |  |
| 融点 | -35 °C (lit.) | | 沸点 | 83 °C (lit.) | | 比重(密度) | 1.256 g/mL at 25 °C (lit.) | | 蒸気密度 | 3.4 (20 °C, vs air) | | 蒸気圧 | 87 mm Hg ( 25 °C) | | 屈折率 | n20/D 1.444(lit.) | | 闪点 | 60 °F | | 貯蔵温度 | 0-6°C | | 溶解性 | 7.9g/l | | 外見 | Liquid | | 色 | APHA: ≤10 | | Relative polarity | 0.327 | | 臭い (Odor) | Chloroform-like odor | | 爆発限界(explosive limit) | 6.2-15.9%(V) | | 水溶解度 | 8.7 g/L (20 ºC) | | Merck | 14,3797 | | BRN | 605264 | | Henry's Law Constant | 11.24 at 30 °C (headspace-GC, Sanz et al., 1997) | | 暴露限界値 | TLV-TWA 10 ppm (~40 mg/m3) (ACGIH),
1 ppm (NIOSH), 50 ppm (MSHA and
OSHA); ceiling 2 ppm/15 min (NIOSH);
carcinogenicity: Animal Sufficient Evidence,
Human Limited Evidence (IARC). | | Dielectric constant | 10.7(20℃) | | 安定性: | Volatile | | LogP | 1.45 at 20℃ | | CAS データベース | 107-06-2(CAS DataBase Reference) | | IARC | 2B (Vol. 20, Sup 7, 71) 1999 | | NISTの化学物質情報 | Ethane, 1,2-dichloro-(107-06-2) | | EPAの化学物質情報 | 1,2-Dichloroethane (107-06-2) |
| | 1,2-ジクロロエタン Usage And Synthesis |
| 外観 | 無色澄明の液体 | | 性質 | 1,2-ジクロロエタンの融点は-98°Cで、沸点は57°Cです。常温では油状であり、エーテル臭がする無色の液体です。1,2-ジクロロエタンは、水にはわずかしか溶けません。、エーテルには溶けます。高い引火性や発癌性の可能性を有します。 | | 反応 | 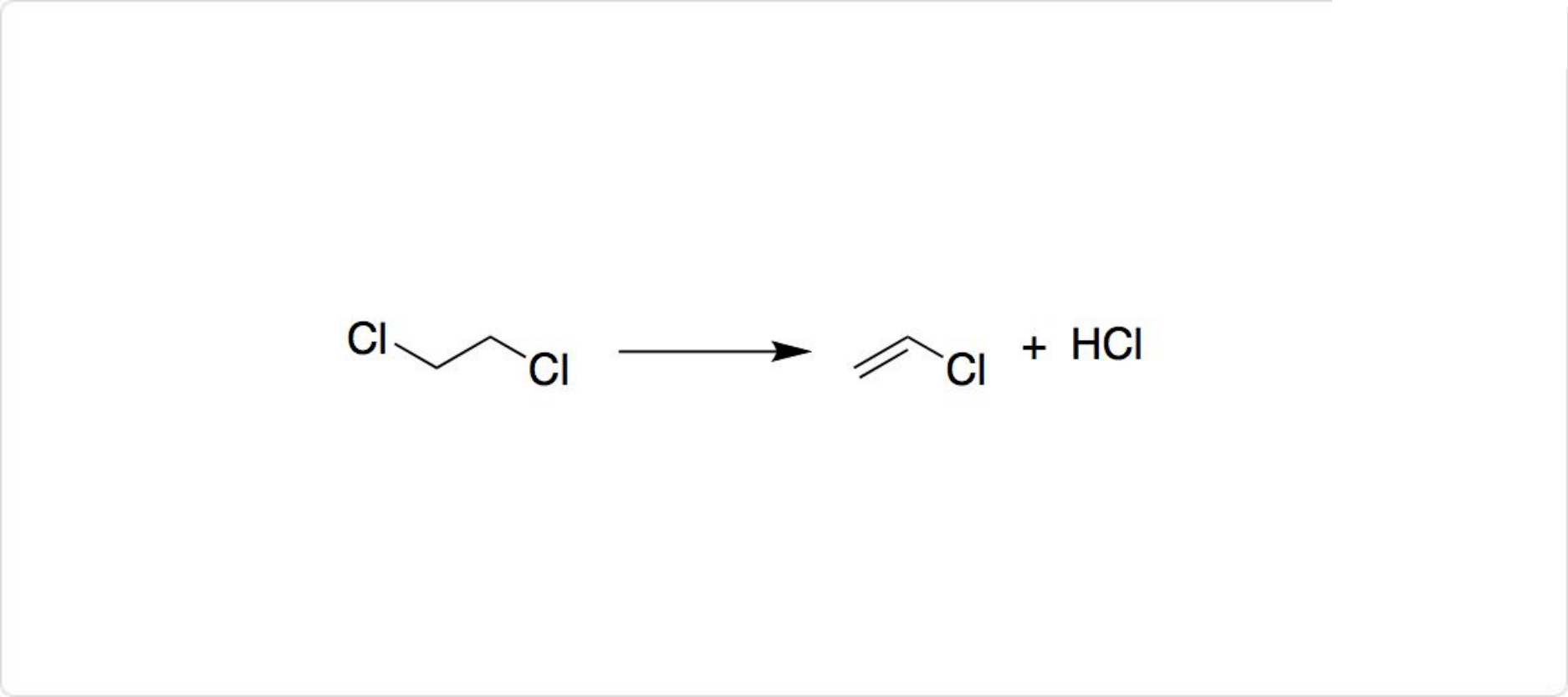
1,2-ジクロロエタンの反応
1,2-ジクロロエタンの生産量の8割が、モノマーである塩化ビニルの生産に使われています。具体的には、1,2-ジクロロエタンから塩化水素が脱離して、ポリ塩化ビニルの前駆体であるクロロエチレン (英: chloroethylene) を得ることが可能です。
クロロエチレンの化学式はCH2=CHClであり、塩化ビニル (英: vinyl chloride) とも呼ばれています。クロロエチレンを得る際の副生成物である塩化水素は、1,2-ジクロロエタンを合成するために再使用できます。
そのほか、1,2-ジクロロエタンは、有機合成化学における有用な反応中間体として利用可能です。 | | 溶解性 | 水に難溶 (0.87g/100ml水), アルコール, エーテル等各種有機溶剤と混和。エタノール及びアセトンに溶けやすく、水に溶けにくい。 | | 解説 | 1,2-ジクロロエタン,ジクロロエタン.塩化エチレン,二塩化エチレンともいう.EDCと略記する.脂肪族ハロゲン化炭化水素の一つ.異性体として1,1-ジクロロエタンがあるが,二塩化エチレンというときには,一般に1,2-ジクロロエタンをさす.工業的には,エテンと塩素および酸素からオキシ塩素化法により合成される.2個のCl原子がそれぞれ異なるC原子と結合しているため,トランス形とゴーシュ形の回転異性体をもつ.快香,甘味を有する無色の重い液体.融点-42.0 ℃,沸点83.7 ℃.d204 1.28034.n20D 1.44432.エタノール,エーテルに易溶,水に微溶.エテンを出発原料とする塩化ビニルモノマー製造の中間体で,熱分解(500 ℃)して塩化ビニルを生成する.ほかに溶剤としても用いられる. | | 用途 | 化学物質製造段階の触媒 | | 用途 | 汎用試薬、高純度を要する溶剤等。 | | 用途 | 残留農薬(殺虫剤)。1,2-ジクロロエタン試験における標準液の調製。 | | 用途 | 塩化ビニルモノマー?エチレンジアミン?ポリアミノ樹脂?イオン交換樹脂合成原料、フィルム洗浄剤、有機合成?ビタミン抽出溶剤、殺虫剤、燻蒸剤(NITE初期リスク評価書) | | 用途 | ポリスチレンなど高分子材料のGPC分析用溶離液調製用。 | | 用途 | 汎用試薬、溶剤、有機合成原料。 | | 用途 | 紫外吸収、蛍光分析用溶媒。 | | 用途 | Edman法によるアミノ酸配列分析用溶媒。 | | 構造 | 1,2-ジクロロエタンと1,1-ジクロロエタンの化学式はC2H4Cl2、モル質量は98.96です。1,2-ジクロロエタンの密度は1.253g/cm3で、二塩化エチレン (英: ethylene dichloride) とも呼ばれます。 | | 合成法 | 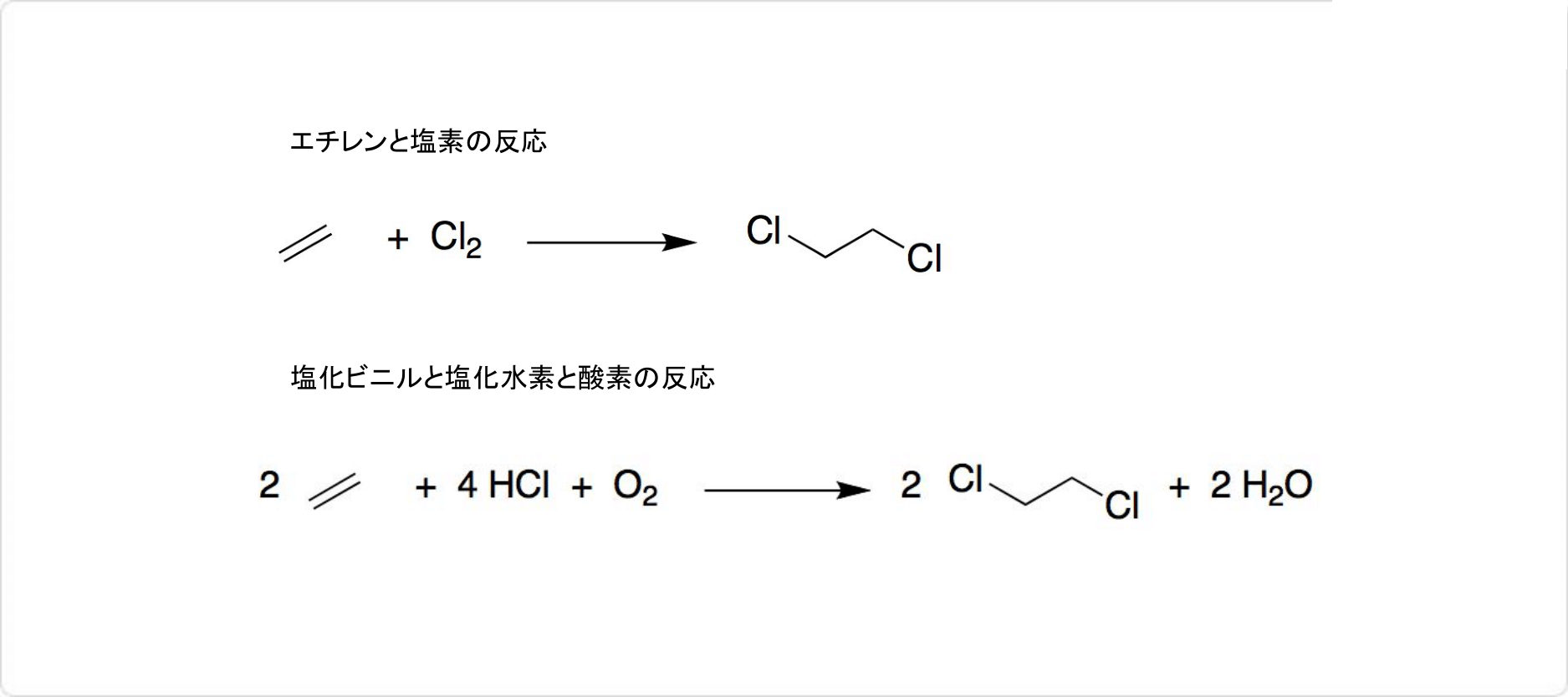
1,2-ジクロロエタンの合成
1,2-ジクロロエタンは、触媒に塩化鉄 (III) を用いて、エチレンとから合成できます。塩化銅 (II) を使用して、塩化ビニル、塩化水素、酸素の反応によっても、1,2-ジクロロエタンが生成します。 | | 使用上の注意 | 不活性ガス封入 | | 説明 | 1,2-Dichloroethane, also called ethylene dichloride (EDC), is a manufactured chemical that is not found naturally in the environment. It is used principally to synthesize vinyl chloride, which is further used to produce a variety of vinyl based plastics products, such as polyvinyl chloride (PVC) pipes, furniture, automobile upholstery, wall coverings, housewares, and automobile parts. It is used in solvents in closed systems for various extraction and cleaning purposes in organic synthesis. It is used as a leaded gasoline additive to remove lead, but with declining tendency. It is used as a dispersant in rubber and plastics, as a wetting and penetrating agent. It was used in ore flotation, as a metal degreaser, and in textile and PVC cleaning. It was also used as an insect fumigant for stored grains and in mushroom houses, a soil fumigant in peach and apple orchards. But due to its toxicity, it is no longer registered for use as an insect fumigant in the United States (IARC 1999).
1,2-Dichloroethane structure | | 化学的特性 | 1,2-Dichloroethane is a clear and colorless, flammable liquid which has a pleasant, chloroform-like odor, and a sweetish taste. Decomposes slowly: turns dark and acidic on contact with air, moisture, and light. The Odor Threshold is 100 ppm.It is a volatile compound. It is relatively insoluble in water (8.6 × 103 mg/l at 25 °C) but soluble in various organic solvents and is miscible with alcohol, chloroform, and ether (NLM, 2013). 1,2-Dichloroethane is one of the highest volume chemicals used in the United States. It is also used as an extraction solvent, as a solvent for textile cleaning and metal degreasing, in certain adhesives, and as a component in fumigants for upholstery, carpets, and grain. | | 物理的性質 | Clear, colorless, oily liquid with a pleasant, chloroform-like odor. The average least detectable
odor threshold concentrations in water at 60 °C and in air at 40 °C were 12 and 52 mg/L,
respectively (Alexander et al., 1982). Experimentally determined detection and recognition odor
threshold concentrations were 25 mg/m3 (6 ppmv) and 165 mg/m3 (41 ppmv), respectively
(Hellman and Small, 1974). | | 使用 | 1,2-Dichloroethane is used in the manufacture of acetyl cellulose and vinyl chloride; inpaint removers; as a fumigant; as a degreaser;as a wetting agent; and as a solvent for oils,waxes, gums, resins, and rubber. It has been used as insect and soil fumigant. | | 主な応用 | 1,2-dichloroethane (Ethylene dichloride), also known as EDC, is produced by reacting chlorine or anhydrous hydrochloric acid with ethylene. The largest single use for EDC is the production of vinyl chloride monomer, which is used to produce poly vinyl chloride (PVC). It has many uses in industry, with principal ones being the following:
As an intermediate in the manufacture of methyl chloroform, perchloroethylene, ethylene amines, polyvinyl chloride (PVC), sulfide compounds, vinyl chloride, and trichloroethane.
As an additive in gasoline (used as a lead scavenger), pharmaceutical products, color film, and pesticides.
As a solvent for rubber, tobacco extract, paint, printing inks, and varnish.
Miscellaneous uses include as an ingredient in fingernail polish, for metal degreasing, in extracting spices, and as a dry cleaning agent. | | 製造方法 | The first synthesis of 1,2-dichloroethane was achieved in 1795. 1,2-Dichloroethane is industrially produced by chlorination of ethylene. This chlorination can either be carried out by using chlorine (direct chlorination) or hydrogen chloride (oxychlorination) as a chlorinating agent. It is also produced by oxychlorination—ethylene, hydrogen chloride, and air are reacted at about 250 °C with a copper chloride catalyst. In the United States, almost all ethylene dichloride produced at present is used as the starting material for preparation of vinyl chloride monomer. | | 定義 | ChEBI: 1,2-dichloroethane is a member of the class of chloroethanes substituted by two chloro groups at positions 1 and 2. It has a role as a non-polar solvent, a hepatotoxic agent and a mutagen. | | 一般的な説明 | Ethylene Dichloride is a clear colorless liquid with a pleasant chloroform-like smell that emits toxic fumes of hydrochloric acid when heated to decomposition. Denser than water and insoluble in water. Vapors are heavier than air. Density 10.4 lb/gal. It is primarily used to produce vinyl chloride. Inhalation exposure to this substance induces respiratory distress, nausea and vomiting and affects the central nervous system, liver and kidneys. It is mutagenic in animals and is reasonably anticipated to be a human carcinogen. (NCI05) | | 空気と水の反応 | Highly flammable. Slightly water soluble. | | 反応プロフィール | Liquid ammonia and 1,2-Dichloroethane can cause an explosion when mixed, NFPA 491M, 1991. A tank of dimethyl amino propyl amine exploded violently when 1,2-Dichloroethane reacted with wet 1,2-Dichloroethane which had been the tank's previous contents [Doyle 1973]. Halogenated aliphatic compounds, such as 1,2-Dichloroethane , are moderately or very reactive. Reactivity generally decreases with increased degree of substitution of halogen for hydrogen atoms. Materials in this group are incompatible with strong oxidizing and reducing agents. Also, they are incompatible with many amines, nitrides, azo/diazo compounds, alkali metals, epoxides, aluminum | | 危険性 | Toxic by ingestion, inhalation, and skin
absorption; strong irritant to eyes and skin; a carcinogen. Flammable, dangerous fire risk, explosive
limits in air 6–16%. Possible carcinogen. | | 健康ハザード | The toxic symptoms from exposure to 1,2-dichloroethane include depression of the cen tral nervous system, irritation of the eyes,corneal opacity, nausea, vomiting, diarrhea,ulceration, somnolence, cyanosis, pulmonaryedema, and coma. Repeated exposure mayproduce injury to the kidney and liver. Inges tion of the liquid can cause death. A fataldose in humans may range between 30 and50 mL. The liquid is an irritant to the skinand damaging to the eyes.
LC50 value, inhalation (rats): 1000 ppm/7 hLD50 value, oral (rabbits): 860 mg/kg
1,2-Dichloroethane tested positive to thehistidine reversion–Ames test and othermutagenic tests. The compound is carcino genic to animals. Inhalation or oral adminis tration caused lung, gastrointestinal, and skincancers in mice and rats. | | 火災危険 | Flammable liquid; burns with a smoky flame;
flash point (closed cup) 13°C (56°F), (open
cup) 18°C (65°F); vapor pressure 62 torr at
20°C (68°F); the vapor is heavier than air and
can travel a considerable distance to a source
of ignition and flash back; autoignition tem perature 413°C (775°F); fire-extinguishing
agent: dry chemical, CO2, or foam; water
may be used to keep fire-exposed contain ers cool and to disperse the vapors and flush
away any spill.
1,2-Dichloroethane forms explosive mix tures with air, with LEL and UEL val ues of 6.2% and 16.0% by volume in air,
respectively. Its reactions with alkali met als, powdered aluminum, or magnesium can
be violent. It forms explosive mixtures with
nitrogen tetroxide. | | 燃焼性と爆発性 | Highly flammable | | 使用用途 | ジクロロエタンは、塩化ビニルモノマー (ビニールの原材料) 、 (塗料、ワニス、、殺虫剤製紙などの原材料) 、ポリアミド樹脂 (プラスチック、の原材料) 、の原材料 (主に水浄化に使用される) などを合成するための原料として使用されます。
また、ジクロロエタンの溶解力は強力なため、非極性非プロトン性溶媒としても有用です。そして、鉄、アルミ、などに付着した油成分を洗浄するための、としても利用できます。
そのほか、塗料の溶剤、スプレー製品の溶剤、毒性を活かして殺虫剤や燻蒸剤 (家屋などのカビ発生防止、害虫の駆除) としての用途もあります。
| | 製品名 | BORER SOL®; BROCIDE®; DESTRUXOL
BORER-SOL®; DOWFUME®[C]; DUTCH LIQUID®;
DUTCH OIL® | | 安全性プロファイル | Confirmed carcinogen with experimental carcinogenic, neoplastigenic, and tumorigenic data. An experimental transplacental carcinogen. A human poison by ingestion. Poison experimentally by intravenous and subcutaneous routes. Moderately toxic by inhalation, skin contact, and intraperitoneal routes. Human systemic effects by ingestion and inhalation: flaccid paralysis without anesthesia (usually neuromuscular blockage), somnolence, cough, jaundce, nausea or vomiting, hypermoulity, diarrhea, ulceration or bleeding from the stomach, fatty liver degeneration, change in cardiac rate, cyanosis, and coma. It may also cause dermatitis, edema of the lungs, toxic effects on the kidneys, and severe corneal effects. A strong narcotic. Experimental teratogenic and reproductive effects. A skin and severe eye irritant, and strong local irritant. Its smell and irritant effects warn of its presence at relatively safe concentrations. Human mutation data reported. | | 職業ばく露 | In recent years, 1,2-dichloroethane is
used in the production of vinyl chloride and as a leadscavenging agent in petrol; it has found wide use in the
manufacture of ethylene glycol, diaminoethylene, polyvinyl
chloride; nylon, viscose rayon; styrenebutadiene rubber,
and various plastics. It is a solvent for resins, asphalt, bitumen, rubber, cellulose acetate; cellulose ester; and paint; a
degreaser in the engineering, textile, and petroleum industries; and an extracting agent for soybean oil and caffeine.
It is also used as an antiknock agent in gasoline; a pickling
agent; a fumigant; and a dry-cleaning agent. It has found
use in photography, xerography, and water softening; and
also in the production of adhesives, cosmetics, pharmaceuticals, and varnishes. | | 応急処置 | If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit.Medical observation is recommended for 24- 48 h afterbreathing overexposure, as pulmonary edema may bedelayed. As first aid for pulmonary edema, a doctor orauthorized paramedic may consider administering a corticosteroid spray. | | 発がん性 | 1,2-Dichloroethane is reasonably anticipated to be a human carcinogen based on sufficient evidence of carcinogenicity from studies in experimental animals. | | Source | Improper use of insecticidal fumigant formulation containing 1,2-dichloropropane and
carbon tetrachloride (Granosan). | | 環境運命予測 | Biological. Methanococcus thermolithotrophicus, Methanococcus deltae, and
Methanobacterium thermoautotrophicum metabolized 1,2-dichloroethane releasing methane and
ethylene (Belay and Daniels, 1987). 1,2-Dichloroethane showed slow to moderate biodegradative
activity with concomitant rate of volatilization in a static-culture flask-screening test (settled
domestic wastewater inoculum) conducted at 25 °C. At concentrations of 5 and 10 mg/L, percent
losses after 4 wk of incubation were 63 and 53, respectively. At a substrate concentration of 5
mg/L, 27% was lost due to volatilization after 10 d (Tabak et al., 1981).
Photolytic. Titanium dioxide suspended in an aqueous solution and irradiated with UV light (λ
= 365 nm) converted 1,2-dichloroethane to carbon dioxide at a significant rate (Matthews, 1986).
The rate constant for the reaction of 1,2-dichloroethane and OH radicals in the atmosphere at
300 K is 1.3 x 10-11 cm3/molecule?sec (Hendry and Kenley, 1979). At 296 K, a photooxidation rate
constant of 2.2 x 10-13 cm3/molecule?sec was reported for the reaction with OH radicals resulting
in a half-life of 1.7 months (Howard and Evenson, 1976).
Chemical/Physical. Anticipated products from the reaction of 1,2-dichloroethane with ozone or
OH radicals in the atmosphere are chloroacetaldehyde, chloroacetyl chloride, formaldehyde, and
ClHCHO (Cupitt, 1980). | | 代謝経路 | Resting cell suspensions of the soil methylotroph
Methylosinus trichosporium OB-3b rapidly
dehalogenate 1,2-dichloroethane, resulting in the
formation of chloroethanol via direct hydroxylation of
one of the C-Cl bonds, and this ethanol is rapidly
oxidized to yield chloroacetic acid. | | 貯蔵 | Ethylene dichloride should be kept protected against physical damage. Store in a cool,
dry, well-ventilated location, away from any area where the fi re hazard may be acute.
Outside or detached storage is preferred. Separate from incompatibles. Containers should
be bonded and grounded for transfer to avoid static sparks. | | 輸送方法 | UN1184 Ethylene dichloride, Hazard Class: 3;
Labels: 3-Flammable liquid, 6.1-Poisonous materials. Note:
United States DOT 49CFR172.101, Inhalation Hazardous
Chemical as 1,2-Dichloroethane | | 純化方法 | It is usually prepared by chlorinating ethylene, so that likely impurities include higher chloro derivatives and other chloro compounds depending on the impurities originally present in the ethylene. It forms azeotropes with water, MeOH, EtOH, trichloroethylene, CCl4 and isopropanol. Its azeotrope with water (containing 8.9% water, and b 77o) can be used to remove gross amounts of water prior to final drying. As a preliminary purification step, it can be steam distilled, and the lower layer is treated as below. Shake it with conc H2SO4 (to remove alcohol added as an oxidation inhibitor), wash with water, then dilute KOH or aqueous Na2CO3 and again with water. After an initial drying with CaCl2, MgSO4 or by distillation, it is refluxed with P2O5, CaSO4 or CaH2 and fractionally distilled. Carbonyl-containing impurities can be removed as described for chloroform. [Beilstein 1 IV 131.] | | Degradation | Ethylene dichloride (1) had been detected in surface and ground water as
an environmental contaminant, resulting from both industrial and
agricultural uses. It is also detected following the chlorination of drinking
water. Ethylene dichloride is stable to hydrolytic degradation at
environmentally relevant pH and temperature.
The primary dissipation mechanism of ethylene dichloride is volatilisation
(DT50 30 min, Moore et al., 1991). Ethylene dichloride interacts with
hydroxyl radicals produced by photo-oxidation in air to yield chloracetyl
chloride (2, Howard and Evenson, 1976; Radding et al., 1977).
The DT50 of ethylene dichloride in the vapour phase under atmospheric
photo-oxidation conditions was 12-122 days (Atkinson, 1985). | | Toxicity evaluation | The estimated daily intake of 1,2-dichloroethane was similar for each rat strain at each dose level. Rats administered drinking water containing 8,000 ppm 1,2-dichloroethane received an esti mated intake of about 500-725 mg/kg per day. This estimated daily intake is close to the report ed oral LDso for 1,2-dichloroethane adminis tered by gavage (680-850 mg/kg) (McCollister et al., 1956); however, intake of this dose over 24 hours rather than as a bolus resulted in little toxicity.
1,2-Dichloroethane toxicity administered by gavage or in formulated drinking water was compared in F344/N rats. Gavage doses were calculated to be approximately equivalent (in milligrams per kilogram) to the range of expo sures resulting from the formulated water mix tures. The F344/N rats were more sensitive to 1,2-dichloroethane administered by gavage than in drinking water, as evidenced by the fact that all males receiving 240 and 480 mg/kg and 9/10 females receiving 300 mg/kg died before the end of the studies.
Toxic encephalopathy is the most common and serious disorder resulting from DCE intoxication. | | 不和合性 | May form explosive mixture with air.
Reacts violently with strong oxidizers and caustics;
chemically active metals, such as magnesium or aluminum powder, sodium and potassium; alkali metals;
alkali amides; liquid ammonia. Decomposes to
vinyl chloride and HCl above 600�℃. Attacks plastics,
rubber, coatings. Attacks many metals in presence of
water. | | 廃棄物の処理 | Incineration, preferably after
mixing with another combustible fuel. Care must be exercised to assure complete combustion to prevent the formation of phosgene. An acid scrubber is necessary to remove
the halo acids produced | | 予防処置 | Occupational workers should avoid use of ethylene dichloride along with oxidizing agents,
strong alkalis, strong caustics, magnesium, sodium, potassium, active amines, ammonia List of Chemical Substances | | 参考文献 | https://www.atsdr.cdc.gov/substances/toxsubstance.asp?toxid=110
[2] http://apps.who.int/iris/bitstream/10665/42027/1/9241530014.pdf
[3] https://www.epa.gov/sites/production/files/2016-09/documents/ethylene-dichloride.pdf |
|