- Vincamine
-

- $1.00 / 1mg
-
2024-04-15
- CAS:1617-90-9
- Min. Order: 100mg
- Purity: 0.99
- Supply Ability: 5ton/month
- Vincamine
-
- $0.00 / 1kg
-
2023-11-27
- CAS:1617-90-9
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 20 tons
- Vincamine
-

- $50.00 / 1kg
-
2023-08-24
- CAS:1617-90-9
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 20 tons
|
| | Vincamine Basic information |
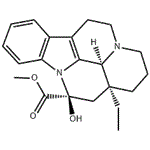
| Product Name: | Vincamine | | Synonyms: | (+)-cis-Vincamine;(3alpha,14beta,16alpha)-14,15-Dihydro-14-hydroxyeburnamenine-14-carboxylic acidmethyl ester;(3alpha,14beta,16alpha)-Dihydro-14-hydroxyeburnamenine-14-carboxylic acid methyl ester;14,15-Dihydro-14-hydroxyeburnamenine-14-carboxylic acid methyl ester;Eburnamenine-14-carboxylic acid, 14,15-dihydro-14-hydroxy-, methyl ester, (3a,14b,16a)-;Oxicebral;Vincamine(8.5%);VINCAMINE(P) | | CAS: | 1617-90-9 | | MF: | C21H26N2O3 | | MW: | 354.44 | | EINECS: | 216-576-3 | | Product Categories: | Inhibitors;OXICEBRAL;API;Alkaloids;Biochemistry;Indole Alkaloids;AlkaloidAsymmetric Synthesis;Biochemicals Found in Plants;Chiral Building Blocks;Complex Molecules;Nutrition Research | | Mol File: | 1617-90-9.mol | 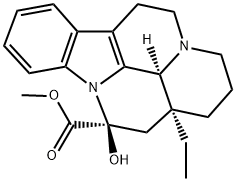 |
| | Vincamine Chemical Properties |
| Melting point | 232 °C (dec.)(lit.) | | Boiling point | 487.66°C (rough estimate) | | alpha | 42.8 º (c=1 in pyridine) | | density | 1.1640 (rough estimate) | | refractive index | 1.6500 (estimate) | | storage temp. | Keep in dark place,Inert atmosphere,2-8°C | | solubility | Chloroform (Slightly), DMSO (Slightly) | | form | Solid | | pka | 12.13±0.40(Predicted) | | color | White to Off-White | | optical activity | [α]23/D +42.8°, c = 1 in pyridine | | Merck | 14,9983 | | Stability: | Hygroscopic | | LogP | 3.100 (est) | | NIST Chemistry Reference | Vincamine(1617-90-9) |
| Hazard Codes | Xn | | Risk Statements | 22 | | Safety Statements | 36-26 | | WGK Germany | 3 | | RTECS | YY8575000 | | HS Code | 29399990 | | Hazardous Substances Data | 1617-90-9(Hazardous Substances Data) | | Toxicity | LD50 in mice (mg/kg): 75 i.v.; >1000 s.c. (Szporny, Szász); 1000 orally (Szabo, Nagy) |
| Provider | Language |
|
(3aS,5S,11S)-3a-Ethyl-5-hydroxy-1,2,3,3a,4,5,10,11b-octahydro-11H-5a,11a-diaza-benzo[cd]fluoranthene-5-carboxylic acid methyl ester
| English |
|
SigmaAldrich
| English |
|
ACROS
| English |
| | Vincamine Usage And Synthesis |
| Chemical Properties | white to almost white fine crystalline powder | | Originator | Pervancamine ,Dausse,France,1969 | | Uses | Vincamine is often used as a nootropic agent to combat the effects of aging, or in conjunction with other nootropics (such as piracetam) for a variety of purposes. Vincamine is a peripheral vasodilator that increases blood flow to the brain. | | Definition | ChEBI: Vincamine is a vinca alkaloid, an alkaloid ester, an organic heteropentacyclic compound, a methyl ester and a hemiaminal. It has a role as an antihypertensive agent, a vasodilator agent and a metabolite. It is functionally related to an eburnamenine. | | Manufacturing Process | The following route is described in US Patent 4,145,552: At ambient
temperature, over a period of thirty minutes, a solution of 33.8g (0.1mol) of
(-)-vincadiformine in a mixture of 140 ml of anhydrous dimethylformamide
and 140 ml of anhydrous toluene is added to a suspension of 2.64 g (0.11
mol) of sodium hydride in a mixture of 200 ml of anhydrous tetrahydrofuran,
20 ml of anhydrous hexamethylphosphotriamide (EMPT) and 18.7 ml (0.14
mol) of trimethyl phosphite. When the release of hydrogen has finished (about
two hours later), the solution is cooled to -10°C and then stirred under an
oxygen atmosphere until absorption ceases (duration: 3 hours). Still at -10°C,
136 ml of glacial acetic acid are added, and the mixture is then left at
ambient temperature for two hours. After the addition of 500 ml of 1 N
sulfuric acid, the aqueous phase is isolated, reextracted with 150 ml of
isopropyl ether, made alkaline with 350 ml of 11 N ammonia, then extracted 3
times with 300 ml aliquots of methylene chloride. After drying over calcium chloride and evaporating the solvent, 30.2 g of crude product are obtained
which, when chromatographed on a column of silica gel (1.5 kg) yield, 9.9 g
of vincamine (yield: 28%) melting point (decomp.): 250°C. | | Brand name | Cerebroxine;Cetal;Ocu-vinc;Oxygeron;Pervincamine;Vadicate;Vinca minor;Vincacen;Vincapront;Vincavix;Vincimax. | | Therapeutic Function | Vasodilator | | World Health Organization (WHO) | Vincamine, an alkaloid derived from Vinca minor, is claimed to
increase cerebral circulation and utilization of oxygen. It is used in a variety of
cerebral disorders and is widely marketed for this purpose. | | General Description | Vincamine is a monoterpenoid indole alkaloid found in the leaves of Vinca minor L., belonging to the Apocynaceae family. |
| | Vincamine Preparation Products And Raw materials |
| Raw materials | Trimethyl phosphite-->Oxygen-->Sodium hydride-->Aspidospermidine-3-carboxylic acid, 2,3-didehydro-, methyl ester, (5alpha,12beta,19alpha)--->(3alpha,16alpha)-D-homoeburnamenine-14,15-dione-->ethyl 3-{[2-(1H-indol-3-yl)ethyl]amino}propanoate-->TABERSONINE-->Methanol-->Tryptamine | | Preparation Products | Vinpocetine-->methyl (3alpha,14alpha,16alpha)-14,15-dihydro-14-hydroxyeburnamenine-14-carboxylate-->EBURNAMENINE-14-CARBOXYLIC ACID, 14,15-DIHYDRO-14-HYDROXY-, ETHYL ESTER, (3A,14B,16A)- |
|