|
|
| | nitrosyl fluoride Basic information |
| Product Name: | nitrosyl fluoride | | Synonyms: | nitrosyl fluoride;Nitrogen oxyfluoride;Nitrosyl fluoride ((NO)F) (9CI) | | CAS: | 7789-25-5 | | MF: | FNO | | MW: | 49.0045032 | | EINECS: | 2321536 | | Product Categories: | | | Mol File: | 7789-25-5.mol | 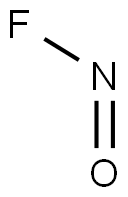 |
| | nitrosyl fluoride Chemical Properties |
| Melting point | -132.5° | | Boiling point | bp -59.9° | | density | (liq at bp) 1.326; d (solid) 1.719 | | form | colorless gas | | color | Colorless gas often bluish because of impurities | | Water Solubility | reacts with H2O to form NO, HNO3, and HF [MER06] |
| | nitrosyl fluoride Usage And Synthesis |
| Chemical Properties | Colorless gas.Attacks glass severely
and corrodes quartz. | | Physical properties | Colorless gas when pure; often appears bluish because of impurities; density 2.176 g/L; liquefies at -56°C; density of liquid 1.326g/mL at its boiling point; solidifies at -134°C; density of solid 1.719 g/cm3; reacts with water. | | Uses | Oxidizer in rocket propellants; stabilizing agent for liquid SO3; fluorinating agent. | | Preparation | Nitrosyl fluoride may be prepared by the reaction of fluorine with nitric oxide:
F2 + 2NO → 2FNO
Nitrosyl fluoride also can be obtained by heating nitrosyl fluborate, NOBF4, and sodium fluoride:
NOBF4 + NaF → NaBF4 + FNO
Nitrosyl fluoborate required for the above preparation may be obtained by dissolving boric acid in 40% HF, concentrating the solution till it fumes, and purifying the NOBF4 formed by sublimation in a vacuum.
Nitrosyl fluoride also can be produced by the action of nitrosyl chloride with silver fluoride:
NOCl + AgF → FNO + AgCl
All preparations must be done in complete absence of water. | | Hazard | Toxic by inhalation, irritant to skin and
mucous membranes. | | Safety Profile | A poison. A severe irritant to skin, eyes, and mucous membranes. Reacts vigorously with glass; corrodes quartz. Explosive reaction with alkenes, oxygen difluoride. Incandescent reaction with metals (e.g., antimony, bismuth,tin, sodium); nonmetals (e.g., a |
| | nitrosyl fluoride Preparation Products And Raw materials |
|