- Camtobell hydrochloride
-

- $3.00 / 3ASSAYS
-
2019-07-10
- CAS:213819-48-8
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 100kg
|
| | Camtobell hydrochloride Basic information |
| Product Name: | Camtobell hydrochloride | | Synonyms: | BELOTECAN HYDROCHLORIDE;CKD-602: BELOTECAN HYDROCHLORIDE;(S)-4-Ethyl-4-hydroxy-11-[2-(isopropylamino)ethyl]-3,4,12,14-tetrahydro-1H-pyrano[3',4':6,7]indolizino[1,2-b]quinoline-3,14-dione hydrochloride;Camtobell hydrochloride;(S)-4-Ethyl-4-hydroxy-11-(2-(isopropylamino)ethyl)-1H-pyrano[3',4':6,7]indolizino[1,2-b]quinol;Belotecan hydrochloride(CKD-602);Belotecan HCl;Belotecan Hydrochlorid | | CAS: | 213819-48-8 | | MF: | C25H28ClN3O4 | | MW: | 469.97 | | EINECS: | | | Product Categories: | Amines;Chiral Reagents;Intermediates & Fine Chemicals;Pharmaceuticals;Amines, Chiral Reagents, Pharmaceuticals, Intermediates & Fine Chemicals | | Mol File: | 213819-48-8.mol | 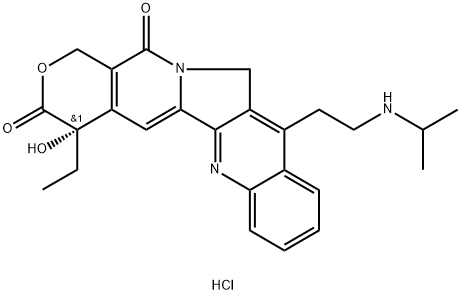 |
| | Camtobell hydrochloride Chemical Properties |
| Melting point | >218°C (dec.) | | storage temp. | under inert gas (nitrogen or Argon) at 2-8°C | | solubility | DMSO (Slightly), Methanol (Slightly, heated), Water (Slightly) | | form | Solid | | color | Pale Beige to Light Brown |
| | Camtobell hydrochloride Usage And Synthesis |
| Description | Camtobell hydrochloride, a DNA topoisomerase I inhibitor, is an analog of camptothecin. It was launched in the Republic of Korea as an injectable formulation for the treatment of ovarian and small cell lung cancer. Although camptothecin exhibits potent antineoplastic activity in vitro, its clinical application is hampered by severe toxicity and poor water solubility. Several synthetic and semi-synthetic analogs of camptothecin with improved solubility and lower toxicity have been developed over the past two decades. Two drugs from this class, topotecan and irinotecan, have been launched in previous years and belotecan is the newest member to reach the market. Camtobell hydrochloride is prepared by a two-step semi-synthesis starting from camptothecin, first by converting to 7-methylcamptothecin via a free-radical methylation reaction using a combination of acetic acid, tert-butylhydroperoxide, ferrous sulfate and sulfuric acid, and subsequently, in the second step, a Mannich reaction with isopropylamine hydrochloride and dimethylsulfoxide. A total synthesis of belotecan in seventeen steps starting from ethyl acetopyruvate is also reported. Belotecan inhibits topoisomerase I with approximately equal potency as camptothecin and about 3-fold higher potency than topotecan, with respective IC50 values of 0.119, 0.123 and 0.33 μg/mL. Its cytotoxic activity is comparable to that of camptothecin, with IC50 values ranging from 2 ng/mL to 2 μg/mL against 26 different human cancer cell lines. In studies using human tumor xenografts in nude mice, 80-100 mg/ kg of belotecan dosed every four days for four doses produced 67 to 94% tumor regression rates against HT-29, WIDR and CX-1 colon, LX-1 lung, MX-1 breast and SKOV-3 ovarian carcinomas. Pharmacokinetic studies of camtobell hydrochloride in rats at intravenous doses of 2.6–8.9 mg/kg demonstrated that both Cmax and AUC increased in a dose-dependent manner. Total clearances, volumes of distribution and mean residence times did not change significantly with increasing doses. The elimination half-life ranged between 9.2 to 11.2 hours. In a Phase I study of camtobell hydrochloride, the fraction of renal clearance was found to be 33.1 to 50.3%, and the protein-binding fraction was 53 to 87%. Approximately 9.5% of the administered dose was excreted via the hepatobiliary system. In clinical studies involving 20 patients with recurrent or refractory ovarian cancer, intravenous administration of 0.5 mg/m2/day of belotecan for 5 days every 3 weeks over a median of six dosing cycles resulted in an overall response rate of 45%. All patients had grade 3 or 4 neutropenia as the most significant adverse event. | | Originator | CKD Pharmaceuticals (S. Korea) | | Uses | A novel camptothecin-derivative anti-tumor agent. CKD-602-related toxicities induced by IV infusion administration have not yet been evaluated, although the drug is more widely used in clinical settings. | | Brand name | Camtobell | | Synthesis | The total synthesis route is depicted in the Scheme. The
known pyridinone 7 was converted to the bicyclic
pyridinone 8 by treatment with methyl acrylate and K2CO3
in DMF. Hydrolysis and decarboxylation of 8 to ketone 9
was effected by refluxing in a mixture of HOAc and conc.
HCl under nitrogen. Ketalization was performed in a two
phase system of toluene and ethylene glycol to provide ketal
10 in 90% yield. Functionalization of the methyl group in
10 using diethyl carbonate in the presence of KH furnished
the ester 11 in 76% yield. Ethylation of 11 was
accomplished by use of KOBut and EtI in DME. Catalytic
hydrogenation of 12 using Raney Ni in a mixture of Ac2O
and HOAc gave the amide 13. Removal of the catalyst by
filtration followed by addition of NaNO2 to the filtrate gave
the N-nitroso amide. Decomposition of the nitroso amide by heating in an inert solvent (CCl4) gave the acetate 14.
The diester 14 was lactonized by LiOH in MeOH/H2O to
give lactone 15 in 92% yield. The carbonyl group in 15
was then reduced with DIBAL-H in THF to give lactol,
which was dehydrated via its mesylate to afford 16. The
asymmetric dihydroxylation of 16 gave diasteromeric
mixtures in favor of the desired isomer 17 (81% d.e.).
Compound 16 was then oxidized directly with iodine in the
presence of CaCO3 to give a-hydroxy lactone 18. The
deketalization was accomplished by HCl in THF/H2O to
provide the ketone 19. Condensation of ketone 19 and
the amine 20 in the presence of p-TSA followed by
hydrolytic removal of Cbz group provided the free base
Which was convert to its corresponding HCl salt as
belotecan hydrochloride (II). 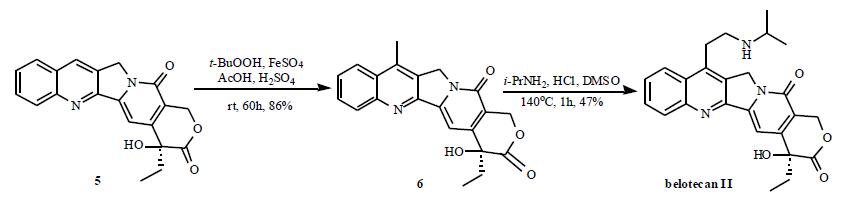
|
| | Camtobell hydrochloride Preparation Products And Raw materials |
|