- Picloram
-

- $200.00 / 1kg
-
2023-06-26
- CAS:1918-02-1
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 1000kg/Month
- Picloram
-

- $0.00 / 25KG
-
2023-06-20
- CAS:1918-02-1
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 50000KG/month
- Picloram
-

- $1.00 / 10g
-
2022-12-29
- CAS:1918-02-1
- Min. Order: 10g
- Purity: 99.5%
- Supply Ability: 1000kg
|
| | Picloram Basic information |
| Product Name: | Picloram | | Synonyms: | RARECHEM AL BO 1809;PICLORAM;OTAVA-BB BB5110090065;4-aminotrichloropicolinicacid;IFLAB-BB F3055-0927;PICLORAM, 250MG, NEAT;4-AMINO-3,5,6-TRICHLOROPICOLINIC ACID, T ECH.;PICLORAM PESTANAL (4-AMINO-3,5,6-TRICHLO | | CAS: | 1918-02-1 | | MF: | C6H3Cl3N2O2 | | MW: | 241.46 | | EINECS: | 217-636-1 | | Product Categories: | INSECT HORMONE | | Mol File: | 1918-02-1.mol | 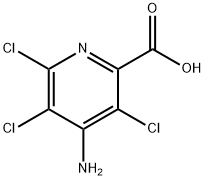 |
| | Picloram Chemical Properties |
| Melting point | 200 °C (dec.)(lit.) | | Boiling point | 421℃ | | density | 1.9163 (rough estimate) | | refractive index | 1.6770 (estimate) | | Fp | >110°(230°F) | | storage temp. | 0-6°C | | solubility | Soluble in acetone | | form | Granules | | pka | 4.1(at 25℃) | | color | White, tan | | Water Solubility | 420 mg/L | | λmax | 252nm(Phosphate buffer sol.)(lit.) | | Merck | 14,7397 | | BRN | 479075 | | Exposure limits | OSHA PEL: 15 mg/m3 (total), 5 mg/m3 (respirable fraction); ACGIH
TLV: TWA 10 mg/m3, STEL 20 mg/m3. | | InChIKey | NQQVFXUMIDALNH-UHFFFAOYSA-N | | LogP | 0.300 | | CAS DataBase Reference | 1918-02-1(CAS DataBase Reference) | | IARC | 3 (Vol. 53) 1991 | | NIST Chemistry Reference | 2-Pyridinecarboxylic acid, 4-amino-3,5,6-trichloro-(1918-02-1) | | EPA Substance Registry System | Picloram (1918-02-1) |
| Hazard Codes | Xi | | Risk Statements | 36 | | Safety Statements | 26 | | RIDADR | 3077 | | WGK Germany | 2 | | RTECS | TJ7525000 | | TSCA | Yes | | HazardClass | 9 | | PackingGroup | III | | HS Code | 2933399990 | | Hazardous Substances Data | 1918-02-1(Hazardous Substances Data) | | Toxicity | LD50 in rats, mice, rabbits, guinea pig, chickens, sheep, cattle (mg/kg): 8200, 2000-4000, 2000, 3000, 6000, >1000, >750 orally (Mullison) |
| | Picloram Usage And Synthesis |
| Description | Picloram is a colourless crystal. It is very soluble in acetone, ethanol, benzene, and dichloromethane.
It is a systemic herbicide used for general woody plant control, sold under the
trade names Tordon and Grazon. It also controls a wide range of broad-leaved weeds, but
most grasses are resistant. It is used in formulations with other herbicides such as bromoxynil,
diuron, 2,4-D, MCPA, triclorpyr, and atrazine. It is also compatible with fertilisers.
Picloram, in the pyridine family of compounds, is a systemic herbicide used for control of
woody plants and a wide range of broad-leaved weeds. Most grasses are resistant to picloram,
so it is used in range management programs. Picloram is formulated either as an acid
(technical product), a potassium or triisopropanolamine salt, or an isooctyl ester, and is
available as either soluble concentrates, pellets, or granular formulations. The materials
in this document refer to the technical acid form unless otherwise indicated. Picloram
is stable under acidic, neutral and basic conditions. Picloram is formulated either as an
acid (technical product), a potassium or triisopropanolamine salt, or an isooctyl ester, and
is available as either soluble concentrates, pellets, or granular formulations and related
manufacturing impurities. | | Chemical Properties | Picloram is a colorless powder. Chlorine odor. | | Uses | Herbicide. | | Uses | It is used as a herbicide and defoliant. | | Uses | Systemic herbicide used to control most broad-leaved weeds on grassland and
noncrop areas. Use as a pesticide is restricted | | Uses | Picloram is a dicot-selective, persistent herbicide and in salt
form is used to control a variety of annual weeds on crops,
perennial broadleaved herbs, and woody species in combination
with 2,4-D or 2,4,5-T. It can persist in an active form in
the soil from several months to years, and can also be released
from the roots of treated plants into the soil, where other nontarget
species may take it up and die. Picloram is of great use
in the management of unwanted vegetation in rangeland,
grass pastures, and forestry as well as non-cropland and rightsof-
way sites, such as around industrial and military installations,
roads, railways, airports, under power lines, and along
pipelines. Additional uses in some countries include in rice,
sugarcane, cereals, and oilseed rape. | | Definition | ChEBI: Picloram is a pyridinemonocarboxylic acid that is pyridine-2-carboxylic acid which is substituted by a chloro group at positions 3,5 and 6, and by an amino group at position 4. It is a systemic herbicide used to control deeply rooted herbaceous weeds and woody plants in rights-of-way, forestry, range lands, pastures, and small grain crops. It has a role as a herbicide and a synthetic auxin. It is an aminopyridine, a pyridinemonocarboxylic acid, a chloropyridine and an organochlorine pesticide. It is functionally related to a picolinic acid. | | General Description | Fine beige crystals or white powder. Odor of chlorine. | | Air & Water Reactions | Insoluble in water. | | Reactivity Profile | Picloram may be sensitive to prolonged exposure to light. Aqueous solutions may be decomposed by light. Picloram is incompatible with strong oxidizing agents, strong acids, acid chlorides and acid anhydrides. | | Health Hazard | The toxic effects from ingestion or inhalation of dusts of picloram in test animals were mild. The acute oral LD50 values inrats and rabbits are 2900 and 2000 mg/kg,respectively. Maternal toxicity in rats wasobserved at a dose level of 750 mg/kg/day.Oral administration of picloram in rats andmice caused tumors in thyroid and liver. | | Fire Hazard | Flash point data for Picloram are not available; however, Picloram is probably combustible. | | Agricultural Uses | Herbicide: Picloram is a systemic herbicide used for control of woody plants and a wide range of broad-leaved weeds along roads, power lines and long right-of-ways. Most grasses are resistant to picloram, so it is used in range management programs to control noxious weeds and brush. It is used to prepare sites for tree planting. Picloram is formulated either as an acid (technical product), a potassium or triisopropanolamine salt, or an isooctyl ester, and is available as either soluble concentrates, pellets, or granular formulations. During the Vietnam war, a herbicide named Agent White was used to control vegetation. It was a mixture of 2,4-D, triisopropanolamine salt and picloram. A U.S. EPA restricted Use Pesticide (RUP). | | Trade name | ACCESS®; AMDON®; AMDON GRAZON®; BOROLIN®; GRAZON® Picloram; K-PIN®; PATHWAY®; TORDON®[C]; TORDON® 101 MIXTURE; TORDON® 10 K; TORDON® 22 K | | Biochem/physiol Actions | Picloram (4-Amino-3,5,6-trichloropyridine-2-carboxylic acid) is a chlorinated systemic herbicide widely used for woody plant and broad-leaved weed control. Picloram induces direct somatic embryogenesis of Lilium longiflorum var. Ceb-dazzel. | | Safety Profile | Moderately toxic by
ingestion. Questionable carcinogen with
experimental carcinogenic, neoplas tigenic,
tumorigenic, and teratogenic data. An
experimental teratogen. Mutation data
reported. When heated to decomposition it
emits very toxic fumes of Cland NOx. | | Potential Exposure | A potential danger to those involved in the manufacture, formulation or application of this herbicide. | | Environmental Fate | Soil. Degrades in soil via cleavage of the chlorine atom at the m-position to form 4-
amino-5,6-dichloro-2-picolinic acid. Replacement of the chlorine at the m-posi-tion by a
hydroxyl group yields 4-amino-3-hydroxy-5,6-dichloropicolinic acid (Hartley and Kidd,
1987). Other soil metabolites reported include carbon dioxide, chloride ions, 4-amino-6-
hydroxy-3,5-dichloropicolinic acid (Meikle et al., 1974), 4-amino-3,5-dichloro-6-hydroxypicolinic acid and 4-amino-3,5,6-trichloropyridine (Goring and Hamaker, 1971). Youngson et al. (1967) reported that degradation increased with an increase in temperature and
organic matter
The half-lives for picloram in soil incubated in the laboratory under aerobic conditions
ranged from 29 days to 3 years with an average of 201 days (Meikle et al., 1973; Yoshida
and Castro, 1975; Merkle et al., 1976). In field soils, the half-lives for pi
Groundwater. According to the U.S. EPA (1986) picloram has a high potential to leach
to groundwater.
Plant. Picloram degraded very slowly in cotton plants releasing carbon dioxide (Meikle
et al., 1966). Metabolites identified in spring wheat were 4-amino-2,3,5-trichloropyridine,
oxalic acid and 4-amino-3,5-dichloro-6-hydroxypicolinic acid (Redemann et al., 1968;
Plimmer, 1970). In soil, 4-amino-3,5-dichloro-6-hydroxypicolinic acid was the only compound positively identified (Redemann et al., 1968)
Photolytic. The sodium salt of picloram in aqueous solution was readily decom-posed
by UV light (λ = 300–380 nm). Two chloride ions were formed for each molecule of
picloram that reacted. It was postulated that degradation proceeded via a free ra | | Metabolism | Chemical. Picloram is generally stable to hydrolytic
degradation but will decompose in hot, concentrated
alkali solutions. It undergoes photodecomposition when
irradiated with UV light and, to a lesser extent,
with sunlight. Degradation via photolysis is thought to
primarily involve cleavage of the ring structure and
liberation of substituent chlorine atoms producing oxamic acid and 3-oxo-β-alanine. Decarboxylation is not thought
to be a major pathway in photolytic degradation.
Plant. Hall et al. (16) have shown that in rapeseed
plants (Brassica spp.) >25% of picloram is metabolized
24 hours after treatment.
Soil. There is only limited microbial degradation in the
soil. If picloram remains on the soil surface, it may undergo
photolysis. | | Shipping | UN2588 Pesticides, solid, toxic, Hazard Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name Required. | | Toxicity evaluation | Mammalian Toxicity. Studies conducted on dog, rat,
steer, and human males indicate that most, if not
all, of orally administered picloram is quickly excreted
unmodified in the urine. The acute oral LD50s for male
rat, mice, rabbit, guinea pig, sheep, and cattle are >5000,
2000–4000, ca. 2000, ca. 3000, >1000, and >750 mg/kg,
respectively.
Weed Resistance/Modified Crop Tolerance. Weed resistance
to picloram has been reported in populations of
yellow starthistle (Centaurea solstitialis) (48) and wild
mustard (Sinapis arvensis) (34). No crops with modified
tolerance toward picloram are currently in production. | | Incompatibilities | Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides. Compounds of the carboxyl group react with all bases, both inorganic and organic (i.e., amines) releasing substantial heat, water and a salt that may be harmful. Incompatible with arsenic compounds (releases hydrogen cyanide gas), diazo compounds, dithiocarbamates, isocyanates, mercaptans, nitrides, and sulfides (releasing heat, toxic and possibly flammable gases), thiosulfates and dithionites (releasing hydrogen sulfate and oxides of sulfur). This material is acidic. Reacts with hot concentrated alkali (hydrolyzes), strong bases. May attack metals. | | Waste Disposal | This chlorinated brush killer is usually formulated with 2,4-D and the disposal problems are similar. Incineration @ 1000�C for 2 seconds is required for thermal decomposition. Alternatively, the free acid can be precipitated from its solutions by addition of a mineral acid. The concentrated acid can then be incinerated and the dilute residual solution disposed in an area where several years’ persistence in the soil can be tolerated. |
| | Picloram Preparation Products And Raw materials |
|